Voided Urine Sample in the Diagnosis of Prostate Cancer in Patients with Serum PSA Ranging between 2.6 to 10 ng/mL
Download
Abstract
Introduction: Prostate cancer (PCa) cells are known to be shed into the prostatic urethra and thus can be collected through a voided urine sample. In this preliminary prospective study, we have assessed the feasibility of detecting cancer of the prostate using a voided urine sample and targeting the genomic VPAC receptors in patients with lower urinary tract symptoms and a serum PSA ranging between 2.6 to 10 ng/mL.
Materials & Methods: Patients ≥40 years old, with lower urinary tract symptoms and serum prostate-specific antigen (PSA) ranging between 2.6 - 10 ng/mL formed the study group. The voided urine sample was collected from all these patients for biomarker testing. All patients underwent a standard 12-core prostate biopsy. The results of the histopathological studies were then compared to the results of the urine biomarker.
Results: A total of 76 male subjects presented to our hospital with lower urinary tract symptoms and a serum PSA ≥ 2.6 and ≤ 10 ng/mL. The voided urine sample was positive for malignant cells in 24/25 (96%) patients with PCa. There was one case of a false negative in this group. Histopathological examination of the core biopsy specimens was positive for malignancy in 23 (92%) cases of PCa.
Conclusions: Voided urine samples can be used to diagnose PCa by targeting the VPAC receptors that are expressed on malignant cells. This test is highly sensitive and serum PSA levels or Gleason’s score have no impact on the sensitivity of this test.
Introduction
Evaluation of healthy asymptomatic men for prostate cancer (PCa) appears to be controversial. There is some disagreement among medical organizations whether the benefits of testing outweigh the potential risks [1]. However discussions regarding the pros and cons of prostate cancer screening could be made if there is a family history of prostate cancer, has other risk factors and if he is a Black person. Prostate cancer screening tests might include a digital rectal examination (DRE) of the prostate and estimation of serum prostate specific antigen (PSA) values.
Prostate-specific antigen (PSA) is a protein secreted by cells of the prostate gland. The chance of having a prostate cancer increases as the PSA level goes up, but there is no set cut-off point that can tell for sure if a person does or doesn’t have prostate cancer. Most men without prostate cancer have PSA levels <4 ng/mL of blood. However, a level <4 ng/mL is not a guarantee of not having a cancer. Men with a PSA level between 4 and 10 ng/mL (often called the “borderline range”) have about a 1 in 4 chance of having prostate cancer. If the PSA is more than 10, the chance of having prostate cancer is over 50% [1]. Abnormalities on DRE or elevated levels of serum PSA is an indication for further tests to be carried out so as to determine if a person has prostatic malignancy, and these include a transrectal ultrasonography (TRUS), Magnetic resonance imaging (MRI) and a prostatic biopsy. TRUS-guided systematic biopsy has been regarded as a standard of care as it was shown to be superior to digitally directed biopsy sampling of the prostate [2]. TRUS biopsy has a false negative rate of 15% - 46% and a tumor under grading rate of up to 38% when compared with the final Gleason score at radical prostatectomy [3]. It has been also shown that random TRUS biopsy detects low grade indolent cancer and this may lead to overtreatment.
Several new biomarkers are being investigated for non-invasive and definitive detection of PCa. Urinary biomarkers are subject of ongoing research and represent a promising alternative or addition to serum-based biomarkers [4]. Prostatic cancer cells are known to be shed into the prostatic urethra and thus can be collected through voided urine sample [5]. These prostate cancer cells could be further imaged optically, by targeting the VPAC1 receptors (combined vasoactive intestinal peptide {VIP} and pituitary adenylate cyclase activating peptide {PACAP} family of cell surface receptors) with the same peptide labelled fluorophore [5,6]. Nerli et al assessed the feasibility of detecting PCa using voided urine samples by targeting the genomic VPAC receptors expressed on the malignant PCa cells in 33 patients with adenocarcinoma. They reported that the results of the biomarker studies and histopathology were consistent with each other. Similarly, Trabulsi et al [6] detected VPAC positive cells in 98.6% of the patients having a PCa diagnosis, (n=141), and none (0%) of the males with benign prostatic hyperplasia (BPH) (n=10). In this preliminary prospective study, we have assessed the feasibility of detecting cancer of the prostate using voided urine sample and targeting the genomic VPAC receptors in patients with lower urinary tract symptoms and a serum PSA ranging between 2.6 to 10 ng/mL.
Materials and Methods
In this prospective study between 1st June 2020 to 31st August 2022, informed consent was obtained from the university/institutional ethical committee prior to initiation of this study. Patients ≥40 years old, with lower urinary tract symptoms and serum PSA ranging between 2.6 - 10 ng/mL formed the study group. Patients with urinary tract infection, haematuria, past history of urothelial carcinoma, radiotherapy were excluded. The first 50 mL of voided urine sample was collected from all these patients for biomarker testing. The urine samples were kept at 22°C and processed at 22°C within four hours of collection.
Processing of Urine samples [5,6]:
The urine samples were centrifuged at 2000xg for 10 minutes and all but approximately 250 μL of supernatant was discarded. The cells were then suspended, and cytocentrifuged, and fixed in 97% ethyl alcohol. TP4303 solution (0.5 μg) was added to the cells to cover the entire cell area, approximately one cm in diameter. The slide was then kept in dark, at 22°C for approximately 20 minutes and then thoroughly rinsed with deionized water and air dried. On the cells was then added, 20 μL of 4′,6-diamidino-2-phenylindole (DAPI, Fisher Scientific, PA) which strongly binds to A-T rich region of DNA in the cell nucleus.
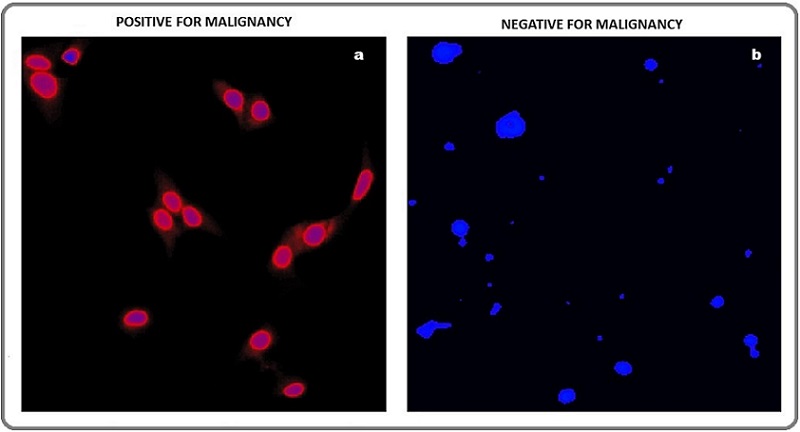
A coverslip was then placed and the slide was observed using a fluorescent microscope. Cells with TP4303 interaction presented themselves with dark orange fluorescence around the nucleus and thereby indicated the presence of VPAC receptor molecules around the cell surface (Figure 1a). In the absence of VPAC receptors, only the DAPI bound cell nucleus was seen in dark blue. Normal epithelial cells that may only have minimal or no expression of VPAC therefore do not interact with TP4303 and show only cell nucleus (Figure 1b).
Figure 1. a. Fluorescence Cytology Showing Positivity for Malignant Urothelial Cells (blue-nuclear and orange-cy- toplasmic fluorescence) Stained with TP4303 and DAPI. b. Fluorescence cytology showing negativity for malignant urothelial cells (absence of orange-cytoplasmic fluorescence) stained with TP4303 and DAPI.
All patients in the study group underwent a standard 12 core prostate biopsy using a transrectal ultrasonography (TRUS) guidance. The specimens were labelled properly denoting the zone of the prostate and sent for histopathological estimation. The results of the histopathological studies were then compared to the results of urine biomarker.
Results
During the study period 1st June 2020 to 31st August 2022, a total of 76 males presented to our hospital with lower urinary tract symptoms and a serum PSA ≥ 2.6 and ≤ 10 ng/mL. The age wise distribution and serum PSA wise distribution was as shown in (Table 1 and 2).
| No | Age | n | Prostate Cancer | Non-malignant |
| 1 | 40-49 | 5 | 2 | 3 |
| 2 | 50-59 | 11 | 5 | 6 |
| 3 | 60-69 | 27 | 7 | 20 |
| 4 | 70 | 33 | 11 | 22 |
| 5 | Total | 76 | 25 | 51 |
| 6 | Mean age years | 69.61± 31.06 | 70.38± 14.91 |
| No | PSA levels ng/mL | n | Prostate Cancer | Non-malignant |
| 1 | 2.6 – 5.0 | 45 | 3 | 42 |
| 2 | 5.1 – 7.5 | 16 | 10 | 6 |
| 3 | 7.6 – 10.0 | 15 | 12 | 3 |
| 4 | Total | 76 | 25 | 51 |
| 5 | Mean serum PSA ng/mL | 4.26± 2.13 | 4.31± 1.75 |
PSA, Prostate specific antigen.
The final diagnosis was cancer of the prostate in 25 patients whereas the remaining 51 were non-malignant cases. The voided urine sample was positive for malignant cells in 24/25 (96%) patients with PCa. There was one case of false negative in this group. When the images of the fluorescent slides were reviewed, there were no cells seen. Of the 51 non-malignant cases, voided urine sample was negative for malignant cells in all (Table 3).
| No | Ca P (25) | Non-malignant (51) | |
| 1 | VPAC receptor positive | 24 (96%) | 0 (0%) |
| 2 | VPAC receptor negative | 1 | 51 (100%) |
| 3 | VPAC False Negative | 4% | - |
| 4 | VPAC False Positive | - | 0% |
| 5 | HPR positive for malignancy | 23 (92%) | 0 (0%) |
| 6 | HPR negative for malignancy | 2 (8%) | 51 (100%) |
| 7 | HPR False negative | 8% | - |
VPAC, (combined vasoactive intestinal peptide {VIP} and pituitary adenylate cyclase activating peptide {PACAP}); HPR, Histopathology report.
Histopathological (HPR) examination of the initial core biopsy specimens was positive for malignancy in 23 (92%) of cases of PCa and were negative for malignancy in all 51 (100%) of non-malignant cases. In the two cases who were negative for PCa on HPR, the voided urine samples were positive for VPAC receptors. Hence a repeat 12 core biopsies were performed, which was positive for PCa (Gleason’s score 3 plus 3).
None of patients with HPR proved PCa had a serum PSA value of <4 ng/mL, whereas 9/51 (17.64%) patients with benign histology had serum PSA > 5 ng/ml. The details of the HPR in the benign cases included 31 (60.78%) with benign prostatic hyperplasia, 15 (29.41%) with chronic prostatitis and the remaining 5 (9.80%) showing a combination of BPH with chronic prostatitis. The Gleason’s score of the PCa patients were as follows, 3+3 in 5 (20%), 3+4 in 14 (56%), and 4+3 in 6 (24%) patients.
Discussion
Prostate cancer at times can be challenging to detect. Presently used screening and diagnostic tools such as prostate-specific antigen testing and transrectal ultrasound-guided prostate biopsy, aren’t perfect. And it becomes all the more difficult when those tools contradict each other. The serum levels of PSA are often elevated in men with prostate cancer, and the PSA test was originally approved by the US Food and Drug Administration (FDA) in 1986 to monitor the progression of prostate cancer in men who had already been diagnosed with the disease.
[7] In 1994, FDA approved the use of the PSA test in conjunction with a digital rectal exam (DRE) to test asymptomatic men for prostate cancer. [7] In addition to prostate cancer, a number of benign (not cancerous) conditions could cause PSA level to rise. The most frequent benign prostate conditions that cause an elevation in PSA level are prostatitis (inflammation of the prostate) and benign prostatic hyperplasia (BPH) (enlargement of the prostate).
A high PSA level and/or an abnormal DRE usually is an indication for prostate biopsy. A TRUS prostate biopsy samples less than 1 percent of the prostate, and the false-negative rate can approach 35 percent, meaning it shows no cancer even though cancer is present [8]. In such instances clinicians are often left with two options namely Multi parametric MRI (MP-MRI) or checking biomarkers. These options can either rule out a clinically significant prostate cancer and potentially avoid a repeat biopsy or indicate an undetected cancer, which may prompt another biopsy and, potentially, treatment.
Multi-parametric magnetic resonance imaging (MP- MRI) has emerged over the years as an imaging test that can improve the accuracy of detecting aggressive prostate cancer. MP-MRI uses multiple, specific imaging sequences instead of just one. MP-MRI can be used to detect suspicious lesions whenever a patient has an elevated PSA level but a negative biopsy. On finding such lesions, one can target them with a biopsy using a unique platform that fuses the previously obtained MRI images with real-time ultrasound either in out-patient clinics or inpatient wards. Standardized interpretation guidelines of MRI, such as the Prostate Imaging Reporting and Data System (PI-RADS version 2) [9, 10], have improved the concordance of radiologists’ reports and interpretation of images, leading to an increase in the prescription of this examination and to the development of targeted TRUS/ MRI fusion biopsies. The results are 89 % accurate whenever the MP-MRI shows no suspicious lesions. 8 Up to 87 % of tumors detected by MP-MRI are considered “clinically significant,” in men with a prior negative biopsy [8].
Biomarkers can also be used whenever the initial biopsy is negative in patients with suspicious lesions on DRE and/or elevated PSA so as to perform or avoid a repeat biopsy. Biomarkers can further indicate whether a certain body process is normal or abnormal. These biomarkers can pinpoint men who actually need a repeat biopsy, as well as help us find more aggressive cancers. 8 Prostate health index (PHI) is a novel approach to improve the clinical performance of PSA wherein the results of three automated blood tests (tPSA, fPSA, and proPSA) are used to derive the term Phi by using a mathematical formula. The Phi test specifically uses the formula (proPSA/fPSA × tPSA) to calculate and report a Phi result, which improves the rate of PCa detection when compared with either tPSA or fPSA/tPSA alone [11]. Lepor et al. [12], after analysing numerous prospective studies from geographically diverse regions concluded that Phi was more specific for PCa detection than existing standard reference tests of tPSA and fPSA. They also pointed out the Phi value was able to predict a greater risk of clinically significant disease on biopsy and an adverse outcome after prostatectomy and further suggested that this test could help monitoring patients on active surveillance.
4K score is a four-kallikrein panel including kallikrein- related peptidase 2 (hK2), intact PSA, fPSA and tPSA. A review of different studies done on the European Randomised Study of Screening for Prostate Cancer (ERSPC) population showed that 4K score increases detection of high-grade cancer and the AUC (area under the curve) between 0.03 and 0.11 ng/mL. According to the cohorts, 2.5%-12% of high-grade cancers were missed [13,14]. This test was then further validated in a prospective multi-institutional study conducted in United States on 1370 men. Twenty-six investigators compared the 4K score with the prostate cancer prevention trial (PCPT RC) 2.0 risk calculator and showed that 4K score was superior to predict Gleason score 7 or more with an AUC of 0.82 vs. 0.74 (p < 0.0001). With a cut-off of 9%, this test could reduce the number of prostate biopsies performed for indolent cancers up to 41% while the diagnosis of Gleason score ≥7 could be missed in 24 men (2.4%) including two patients with Gleason score 4 plus 4 or higher. With a cut-off of 15%, this test could reduce the number of prostate biopsies performed for indolent cancer up to 58% while the diagnosis of Gleason score ≥7 could be missed in 48 men (4.7%) [15].
PCa gene 3 (PCA3) mRNA, which is over-expressed in men with a PCa has been evaluated for guiding biopsy decisions for men with previously negative biopsies and PSA levels persistently >4 ng/mL. As of now, this remains the main indication for this test [16]. Different thresholds have been proposed to improve the sensibility/specificity ratio of this test. Usually, a threshold of 35 has been reported. Chevli et al. [17] in a large study of 3073 men demonstrated that PCA3 was a useful tool in identifying patients at risk for PCa.
Our study clearly shows that PCa can be diagnosed using voided urine sample and by targeting the VPAC receptors. Prostatic cells that appear in the urine are usually luminal epithelial cells that have been shed from the gland. Shedding of prostate cells presumably occurs during normal cell turnover. Identifying prostate cells solely based on morphology is difficult even for trained cyto-pathologists because of their overlapping appearance with other cell types found in the urine sediment as well as their scarcity in regularly voided urine specimens. VPAC receptors are expressed in high density on PCa cells at the onset of oncogenesis and these can be specifically targeted using the fluorophore TP4303 which has been developed by the group in Thomas Jefferson University [6,18, 19]. Our study also showed that irrespective of the low serum PSA values (2.6 – 10 ng/mL) or low Gleason’s grade (3+3), it was possible to identify PCa cells in voided urine samples.
In conclusion, voided urine samples can be used to diagnose PCa by targeting the VPAC receptors that are expressed on malignant cells. Serum PSA levels or Gleason’s score has no impact on the sensitivity of this test. The tests are associated with 0% false positivity rate as well as high (96%) sensitivity rates. This test can be used to indicate a repeat prostatic biopsy in patients with clinically benign appearing prostate with mildly elevated serum PSA and a negative initial TRUS guided biopsy.
Conflict of interest
The authors Madhukar L. Thakur and Leonard Gomella have a conflict of interest towards the patented and patents pending product/TP4303 molecule that is mentioned in the manuscript and is important to the outcome of the study presented.
References
- Tests to Diagnose and Stage Prostate Cancer [Internet] 2021 https://www.cancer.org/cancer/prostate-cancer/detection-diagnosis-staging/how-diagnosed.html. [Cited on 5th October 2021]..
- Random systematic versus directed ultrasound guided transrectal core biopsies of the prostate Hodge KK , McNeal JE , Terris MK , Stamey TA . The Journal of Urology.1989;142(1). CrossRef
- Concordance between Gleason scores of needle biopsies and radical prostatectomy specimens: a population-based study Kvåle R, Møller B, Wahlqvist R, Fosså SD , Berner A, Busch C, Kyrdalen AE , et al . BJU international.2009;103(12). CrossRef
- Tumour markers in prostate cancer III: biomarkers in urine Roobol MJ , Haese A, Bjartell A. Acta Oncologica (Stockholm, Sweden).2011;50 Suppl 1. CrossRef
- Voided urine test to diagnose prostate cancer: Preliminary report Nerli RB , Ghagane SC , Bidi SR , Thakur ML , Gomella L. CytoJournal.2021;18. CrossRef
- Development of a voided urine assay for detecting prostate cancer non-invasively: a pilot study Trabulsi EJ , Tripathi SK , Gomella L, Solomides C, Wickstrom E, Thakur ML . BJU international.2017;119(6). CrossRef
- Prostate-Specific Antigen (PSA) Test. [Internet] 2021 https://www.cancer.gov/types/prostate/psa-fact-sheet. [Cited on 6th October 2021]..
- What to do when prostate cancer biopsy/PSA test results conflict. [Internet] 2017 Krasnow RE , Stamatakis L . https://blog.medstarwashington.org/2017/09/28/prostate-cancer-high-psa-negative-biopsy/ [Cited on 7th October 2021]..
- PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2 Weinreb JC , Barentsz JO , Choyke PL , Cornud F, Haider MA , Macura KJ , Margolis D, et al . European Urology.2016;69(1). CrossRef
- Diagnosis of prostate cancer Descotes J. Asian Journal of Urology.2019;6(2). CrossRef
- A multicenter study of [-2]pro-prostate specific antigen combined with prostate specific antigen and free prostate specific antigen for prostate cancer detection in the 2.0 to 10.0 ng/ml prostate specific antigen range Catalona WJ , Partin AW , Sanda MG , Wei JT , Klee GG , Bangma CH , Slawin KM , et al . The Journal of Urology.2011;185(5). CrossRef
- The Prostate Health Index: Its Utility in Prostate Cancer Detection Lepor A, Catalona WJ , Loeb S. The Urologic Clinics of North America.2016;43(1). CrossRef
- The added value of percentage of free to total prostate-specific antigen, PCA3, and a kallikrein panel to the ERSPC risk calculator for prostate cancer in prescreened men Vedder MM , Bekker-Grob EW , Lilja HG , Vickers AJ , Leenders GJLH , Steyerberg EW , Roobol MJ . European Urology.2014;66(6). CrossRef
- A Systematic Review and Meta-analysis of the Diagnostic Accuracy of Prostate Health Index and 4-Kallikrein Panel Score in Predicting Overall and High-grade Prostate Cancer Russo GI , Regis F, Castelli T, Favilla V, Privitera S, Giardina R, Cimino S, Morgia G. Clinical Genitourinary Cancer.2017;15(4). CrossRef
- A multi-institutional prospective trial in the USA confirms that the 4Kscore accurately identifies men with high-grade prostate cancer Parekh DJ , Punnen S, Sjoberg DD , Asroff SW , Bailen JL , Cochran JS , Concepcion R, et al . European Urology.2015;68(3). CrossRef
- Can urinary PCA3 supplement PSA in the early detection of prostate cancer? Wei JT , Feng Z, Partin AW , Brown E, Thompson I, Sokoll L, Chan DW , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2014;32(36). CrossRef
- Urinary PCA3 as a predictor of prostate cancer in a cohort of 3,073 men undergoing initial prostate biopsy Chevli KK , Duff M, Walter P, Yu C, Capuder B, Elshafei A, Malczewski S, Kattan MW , Jones JS . The Journal of Urology.2014;191(6). CrossRef
- VPAC1 receptors for imaging breast cancer: a feasibility study Thakur ML , Zhang K, Berger A, Cavanaugh B, Kim S, Channappa C, Frangos AJ , Wickstrom E, Intenzo CM . Journal of Nuclear Medicine: Official Publication, Society of Nuclear Medicine.2013;54(7). CrossRef
- Detection of bladder cancer using voided urine sample and by targeting genomic VPAC receptors Nerli RB , Ghagane SC , Rangrez S, Chandra S, Thakur ML , Gomella L. Indian journal of urology: IJU: journal of the Urological Society of India.2021;37(4). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details