Correlation between ALK, ROS1 Biomarkers and EGFR Oncogene Mutations in Lung Tumours: Our Observations in an Apex Oncopathology Laboratory
Download
Abstract
Introduction: Analysis of Anaplastic lymphoma receptor tyrosine kinase gene (ALK), Repressor of Silencing 1 (ROS1) gene are determined by immunohistochemistry (IHC) and it is an easily applicable, cost-effective assay for potential treatment with crizotinib. Mutations of Epidermal growth factor receptor (EGFR) genes are evaluated by IHC/Multiplex RT-PCR. The purpose of this study is to assess the frequencies of ALK, ROS1 and their association with EGFR fusion gene mutations in a spectrum of lung tumours.
Materials and methods: A total of 202 cases of lung tumours reported at our Center for Oncopathology from September 1st 2020 to 31st August 2021, were retrospectively analyzed for ALK, ROS1 and EGFR fusion genes based on Immunohistochemistry (IHC) and Multiplex PCR findings. ALK was tested using D5F3 clone, and ROS1 was analyzed using Cell Signalling’s D4D6 clone on the Ventana immunohistochemistry platform. EGFR status was analyzed using EGFR mutation test V2 real-time multiplex PCR assay on Roche Cobas Z480.
Results: 202 biopsy samples and cellblocks of fluid aspirates were analyzed. 175/202 were histologically and immunologically proved as Non-small cell lung carcinoma (Primary pulmonary adenocarcinoma) and its metastases. 09/199 (5.23%) were Positive for ALK IHC and 03/199 (1.74%) cases had equivocal results. 06/179 (3.85%) cases were Positive for ROS1 IHC and 03/179 (1.92%) cases had equivocal results. Other histo-morphological diagnoses i.e., adenosquamous, squamous, small cell, mucinous carcinoma etc (27 cases) were all ALK and ROS1 Negative. 188/202 tumours were analyzed for EGFR mutation status, which showed 70/188 (37.23%) had specific EGFR mutations. 118/188 (62.76%) cases were EGFR wildtype.
Conclusion: We observed that age related incidence of EGFR mutations was highest in elderly females, of 61 to 70 years. ALK gene mutations occurred in 6.03% and ROS1 gene mutations occurred in 5.02% of lung tumours and their metastases. EGFR-mutations were associated with ROS1 mutated lung adenocarcinomas. There are no coexistent ALK-EGFR or ALK-ROS1 mutations. All ALK IHC positive pulmonary adenocarcinomas are ROS1 negative and are mutually exclusive.
Introduction
Lung cancer remains one of the most common leading causes of cancer-related deaths worldwide accounting for 13% of total cancer cases. Lung cancer is the commonest malignancy in males, most commonly associated with or without smoking history [1]. Anaplastic Lymphoma Kinase (ALK) gene rearrangement was identified in non-small cell lung carcinomas as an inversion in chromosome 2p with or without interstitial deletion, resulting in the echinoderm microtubule-associated protein-like 4 (EML4)–ALK fusion product [2]. Repressor of Silencing 1 (ROS1) is a receptor tyrosine kinase on long arm of chromosome 6, which is part of the insulin receptor family. Breakpoint mutations and translocations of ROS1 activates the STAT3, PI3kinase, as well as the RAS/RAF/MAPKpathway by phosphorylation of RAS [3].
In patients with advanced lung adenocarcinomas, testing for somatic mutations and rearrangements in EGFR, ALK and ROS1 gene is now mandated as part of normal pretreatment screening. In comparison to patients receiving standard platinum doublet chemotherapy, patients in these molecularly defined subsets have better and more long-lasting outcomes, less toxicity, and a higher quality of life when combined with the proper oral tyrosine kinase inhibitors (TKIs) [4,5].
ALK and ROS1 rearrangements have been identified as a distinct subset of Non-small cell lung carcinoma(NSCLC) in 3–5% and 1–2% respectively, which have also shown sensitivity to treatment with the tyrosine kinase inhibitor, Crizotinib with excellent response rates [5].
Materials and Methods
Data Collection
All tumor resection and biopsy specimens followed at Center for Oncopathology, Mumbai, with a diagnosis of lung cancer were recorded through an ongoing institutional review study. Data was collected manually from the patient’s medical records and by using Creliohealth electronic data capture at Center for Oncopathology, retrospectively from September 1st 2020 to 31st August 2021.
Automated IHC for ALK and ROS1 expression was performed for all cases on Roche Ventana BenchMark- XT instrument. FFPE tumor tissues were sectioned at a thickness of 5.0 mm and stained with D5F3 (Companion diagnostic assay [6]) and D4D6 (Cell Signaling Technology [3]) clones respectively, with the OptiView DAB IHC Detection Kit and OptiView Amplification Kit, as per the manufacturer’s instructions.
Histopathological type, tumor genotype, ALK, ROS1, EGFR gene statuses and clinical characteristics were retrospectively extracted from medical records based on Immunohistochemistry (IHC) and Multiplex PCR findings.
ALK1 IHC Interpretation (Table 1).
| Clinical Interpretation | Staining Description |
| Positive for ALK | Presence of strong granular cytoplasmic staining in tumor cells (any percentage of positive tumor cells). |
| Certain staining elements should be excluded, including: | |
| • Light cytoplasmic stippling in alveolar macrophages | |
| • Cells of neural origin (nerve and ganglion cells) | |
| • Glandular epithelial staining | |
| • Scattered lympho-reticular cells within lymphocytic infiltrate. | |
| Some background staining also may be observed within normal mucosa in NSCLC (including mucin) and in necrotic tumor areas, which should be excluded from the clinical evaluation. | |
| Negative for ALK | Absence of strong granular cytoplasmic staining in tumor cells. |
ROS1 IHC interpretation:
The final staining intensity result was defined as follows: [7,8]
Strong cytoplasmic staining (3+), which was clearlyb visible with use of a 2x or 4x objective;
Moderate staining (2+), which required use of a 10x or 20x objective;
Weak staining (1+), which involved use of a 40x objective; and Negative staining (0), lack of expression.
The percentages of tumor cells with varying staining intensity were evaluated. Membrane staining was recorded when observed. ROS1 IHC staining results were finally interpreted by using four previously described criteria:
(1) an H-score with a threshold for ROS1 positivity defined as at least 100,
(2) an H-score cutoff of at least 150,
(3) an intensity criterion with the cutoff of positivity defined as 2+ or higher in any tumor cells &
(4) a positive status based on an intensity of 2+ or higher in at least 30% of the total tumor cells.
Intratumoral staining heterogeneity was also evaluated. It was defined as the presence of areas of staining with an intensity of 0 or 1+ in positive cases [8]. The positivity of normal lung tissue was recorded when it was present on the sections.
EGFR status was analyzed using EGFR mutation test V2 real-time multiplex PCR assay on Roche Cobas Z480 [9] and is designed to detect the following mutations:
Exon 18: G719X (G719A, G719C, and G719S)
Exon 19: deletions and complex mutations (combination of deletion and insertion) Exon 20: S768I, T790M and insertions Exon 21: L858R and L861Q Mutation.
Each run included a mutant control and a negative control to ensure its validity.
Inclusion Criteria
All USG, EBUS and CT guided core needle biopsies, resection specimens and paraffin embedded formalin fixed tissue blocks received from September 1st 2020 to 31st August 2021, in proper fixative with provisional clinical diagnosis of Lung Carcinomas and its metastases are included, irrespective of age, gender and histological diagnosis.
Exclusion Criteria
The cases without a clinical diagnosis of lung carcinoma and distant metastases occurring in lungs from primary carcinomas arising in distant organs other than the lungs are excluded.
Statistical Methods
For categorical variables, statistical analysis was done using either the Fisher’s exact test or the Pearson Chi-square test. A two sided P < 0.05 was considered significant, and tests were evaluated using SPSS Software version 24.
Results
Among 202 lung tumors tested, 126 were male and 76 were female, youngest being 29 years female and most elder being 87 years male. The age related incidence was highest in age range of 61 to 70 years (Figure 1).
Figure 1. Age Related Incidence of Lung Carcinomas.
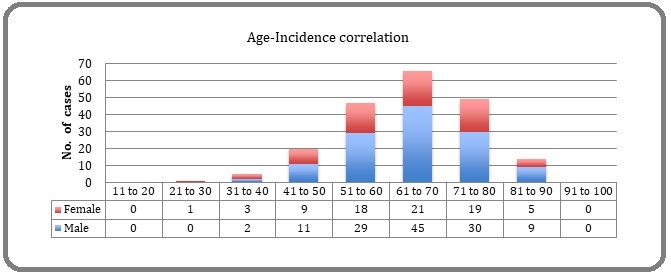
A total of 202 surgical resection specimens, small biopsy (trans-bronchial or core needle) samples, and cell-blocks of needle aspirates/pleural fluid received were analyzed. 172/202 cases were histologically and immunologically proved as NSCLC (Adenocarcinoma) and its metastases.
Apart from histological diagnosis of pulmonary adenocarcinoma and its metastasis in 175 tumours, 13 were squamous cell carcinoma, 3 were adenosquamous carcinoma, 2 were TTF-1 negative poorly-differentiated adenocarcinoma, 2 were mucinous adenocarcinoma, 2 were small cell carcinoma, 3 were sarcomatoid carcinoma and 1 was malignant spindle cell tumour and 1 fibro-inflammatory tumor respectively. All these tumours were Negative for ALK and ROS1 on immunohistochemistry. 3 tumours were not tested for ALK as the treating physician requested only for ROS1/ EGFR mutation status (Table 2).
| Histological Diagnosis | Total No of cases | ALK | ROS1 | EGFR mutated (out of 22 cases) | ||||||
| Total Tested | Pos | Eqv | Neg | Total Tested | Pos | Eqv | Neg | |||
| Pulmonary Adenocarcinoma | 175 | 172 | 9 | 3 | 160 | 155 | 6 | 3 | 146 | 66/167 |
| Adenosquamous carcinoma | 3 | 3 | 0 | 0 | 3 | 3 | 0 | 0 | 3 | 1/2 |
| Squamous carcinoma | 13 | 13 | 0 | 0 | 13 | 10 | 0 | 0 | 10 | 2/9 |
| Small cell carcinoma | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 1/2 |
| Sarcomatoid Carcinoma | 3 | 3 | 0 | 0 | 3 | 3 | 0 | 0 | 3 | 0/3 |
| TTF-1 Negative Adenocarcinoma | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 0/2 |
| Mucinous Adenocarcinoma | 2 | 2 | 0 | 0 | 2 | 2 | 0 | 0 | 2 | 0/2 |
| Fibro-inflammatory tumour of lung | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0/0 |
| Malignant Spindle cell tumour | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0/1 |
| Total No of cases | 202 | 199 | 9 | 3 | 187 | 179 | 6 | 3 | 170 | 188 |
| Cases Not Tested | 3 | 23 | 14 | |||||||
| Percentage (%) | 6.03% | 5.02% | 37.23% |
Pos, Positive; Neg, Negative; Eqv, Equivalent
All the equivocal ALK and ROS1 IHC positive tumours are considered positive for statistics as intensity criterion with the cutoff of positivity is defined as 2+ or higher in any tumor cells [6,7,10].
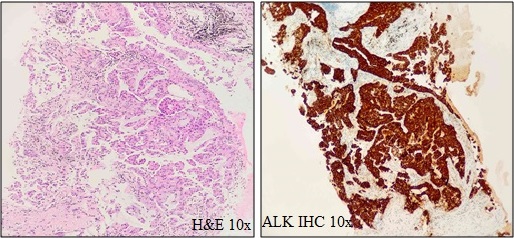
199 cases were tested for ALK IHC, out of which 9 were 3+ Positive and 3 cases were 2+ positive results. (Total = 6.03%) (Figure. 2–H&E and –ALK IHC–10X).
Figure 2. H&E and ALK IHC Showing Homogeneous Strong Granular Cytoplasmic Positivity.
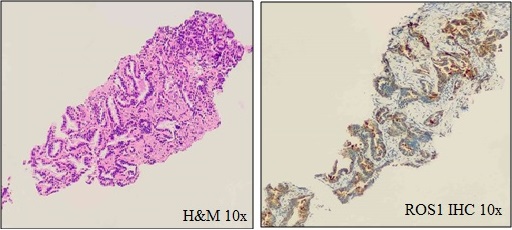
179 cases were tested for ROS1 IHC, out of which 6 cases were Positive and 3 cases had equivocal results. (Total = 5.02%) (Figure 3–H&E and –ROS1 IHC–10X).
Figure 3. H&E and ROS1 IHC Showing Strong Cytoplasmic (3+) Positivity in Low Power.
All ROS1 positive cases had Exon 19, Exon 21– L858R mutations (5/6 and 1/6 respectively) and 3 equivocal ROS1 positive cases tested positive for Exon 19 mutation, showing moderate association (p < 0.1) between Exon 19 EGFR mutation and ROS1 with 62.13% accuracy (p value = 0.08).
On the contrary, All ALK positive cases had Wildtype EGFR gene status demonstrating that ALK and EGFR mutations have no association with mutual exclusivity (p value = 0.03) in the cases we reviewed.
All tumours showing ALK positivity/equivocal are negative for ROS1 mutations and all tumours positive/ equivocal for ROS1 are negative for ALK mutations on IHC.
188 out of 202 tumours were analyzed for EGFR mutation status, which showed 70 (37.23%) lung tumours had EGFR mutation. 48 (25.5%) tumours were positive for Exon-19 deletions and complex mutations (including 1 Squamous Cell Carcinoma and 1 Small cell Carcinoma), Exon-20 mutations in 5 (2.65%) tumours (insertion in 1, T790M mutation in 1, point mutations in 3 tumours), Exon-21 (L858R) mutation in 17 (9.04%) tumours (including 1 case of Squamous Cell Carcinoma and 1 case of Adenosquamous Carcinoma). 118 cases out of 188 (62.76%) tested were EGFR wildtype.
Discussion
NSCLC is the largest subgroup of lung cancer harboring a majority of activating mutations and rearrangements in chromosome 2, 6 and 7. Recently discovered echinoderm microtubule-associated protein- like 4-anaplastic lymphoma kinase (EML4-ALK) rearrangement by Soda et al., is sensitive to the ALK inhibitors [2]. Targeting these mutations has changed the overall treatment strategy for lung cancer and provides significant benefit to the patient. In oncology clinical practice, EGFR mutation screening is emerging into a standard of care, and treatment with inhibitors increased survival for patients with lung cancer with an EGFR mutation. In this paper, we reported the association of ALK, ROS1 and EGFR mutation in lung cancer in India with the data from an apex oncopathology lab.
ALK
The anaplastic lymphoma kinase (ALK) protein is a member of the insulin receptor superfamily of receptor tyrosine kinases. ALK is a type I membrane glycoprotein that is normally expressed in the nervous system. The ALK gene resides at chromosome 2p23 and is constructed of 2 large introns and 26 exons [11].
ALK rearrangements in NSCLC were first discovered in 2007 when a fusion was demonstrated between ALK and echinoderm microtubule-associated protein- like 4 (EML4) due to an inversion on the short arm of chromosome 2 [2,12]. EML4 is a widely expressed cytoplasmic protein involved in the formation of microtubules. These ALK fusions in NSCLC are usually a result of an intra-chromosomal rearrangement in which the intracellular kinase domain of ALK fuses to the N-terminal of the EML4 gene [2]. There are numerous known ALK-EML4 variations that all arise from the same region of the ALK gene but involve various breakpoints in the EML4 gene. Other uncommon fusion partners include a member of the kinesin family 5B (KIF5B), a TRK-fused gene (TFG), and kinesin light chain 1 (KLC1) [12-14].
The product of EML4-ALK fusion is a chimeric protein with constitutive ALK activity and is detected in 3–6% of unselected NSCLC and especially among never-smokers or light ex-smokers who have adenocarcinoma histology [15].
ALK-positive NSCLC represents a distinct molecular subtype that can be targeted with ALK-specific treatments [2,12]. Crizotinib is an oral tyrosine kinase inhibitor that targets ALK, MET, and ROS1 tyrosine kinases [16].
In our study, 6. 3% cases of lung cancers (n=199) showed ALK alterations with all cases having Wildtype EGFR gene status. Out of 199 cases tested for ALK IHC (D5F3 clone), 9 were 3+ Positive and 3 cases were 2+ positive results and the remaining are negative.
We observed that ALK and EGFR mutation statues are mutually exclusive, and are in concordance to the studies of Gainor JF et al., and Camidge DR et al [17,18].
ROS1
ROS-1 is an orphan receptor tyrosine kinase which is part of the insulin receptor family, located on chromosome 6q22.1 that is phylogenetically related to ALK [19,20]. ROS1 rearrangements were first described in the glioblastoma cell line U118MG but have since been described in cholangiocarcinoma at a frequency of approximately 9% [20].
ROS1 (chromosome 6q22) encodes a receptor tyrosine kinase that is a member of the insulin receptor family which activates downstream signaling through the mitogen-activated protein kinase pathway, promoting cell growth, proliferation and reducing apoptosis. ROS1 rearrangements were later described in NSCLC in 2007 in the lung cell line HCC78 with fusion partner SLC34A2 and with CD74 [6]. Since then multiple other fusion partners have been identified such as FIG (also known as GOPC), SDC4, TPM3, EZR, and LRIG3 [21]. All ROS1 rearrangements involve the same 3′ region of the kinase domain of the ROS1 gene, which fuse to the 5′ region of the partner genes, i.e., CD74, EZR, SLC24A2, and FIG genes and define a new genomic driver in 1–2.5% of NSCLC patients [10].
Since ROS1-rearranged NSCLC is uncommon and ROS1 fusions must be detected by a complex and time-consuming break-apart FISH experiment, simpler screening techniques (such as immunohistochemistry) and diagnostic algorithms are required to identify patients with ROS1 rearranged NSCLC [22]. Currently, patients (preferably never-smokers) who do not have driver mutations like EGFR, KRAS, HER-2, ALK, RET rearrangements and MET amplifications should be examined for ROS1 fusions since they can get crizotinib-targeted therapy.
The frequency of ROS1 rearrangements ranges from 3.9% to 7.4% in lung adenocarcinoma patients with wild-type EGFR/KRAS/ALK [23]. In our study, 9 (5.02%) cases of lung cancers showed ROS-1 mutations on IHC with D4D6 clone, with associated EGFR; Exon 19, Exon 21–L858R mutations (8/9 and 1/9 respectively), showing moderate association between Exon 19 EGFR mutation and ROS1 with 62.13% accuracy (P value = 0.08). Our observations were in concordance with the findings of Wu S et al and Mescam-Mancini L et al [23].
EGFR
The tyrosine kinase domain exons 19–21 of the EGFR gene, which has 28 exons and is found on the short arm of chromosome 7’s 12–14 region, contain the majority of mutations. The epidermal growth factor receptor family (ERBB family) comprises four tyrosine kinase receptors: HER-1 (EGFR), HER-2/neu (ERBB2), HER- 3 (ERBB3), and HER-4 (ERBB4) [24]. After ligand binding, the EGFR receptors homo- and hetero-dimerize, induce autophosphorylation of the intracellular tyrosine kinase domain, and start a molecular chain of events that contribute to cell growth, proliferation, differentiation, and survival [25]. Simple receptor tyrosine kinase inhibitors (TKIs) bind to the intracellular catalytic domain of the receptor and compete with adenosine triphosphate (ATP) to prevent receptor autophosphorylation and the activation of downstream signaling cascades. The most well researched reversible EGFR-TKIs for people with metastatic NSCLC are gefitinib and erlotinib [26,27]. Before 2004, activating mutations in exon 18, 19, and 21 of the EGFR gene were not known to be the indicator of sensitivity to EGFR-TKIs [28,29]. For over 85% of all mutations, exon 21 point mutations and exon 19 overlapping deletions are causative. Exon 18 and exon 21 both have point mutations that result in amino acid changes, and exon 19 has in-frame deletions that are concentrated around the ATP-binding pocket of the intracellular tyrosine kinase domain. The mutant EGFR has a reduced affinity for ATP in the presence of EGFR-TKI when compared to a wild-type receptor, according to a kinetic investigation of the intracellular domains of the mutant EGFR [30].
Lung cancer adenocarcinoma possesses higher EGFR mutation rates than any other form of NSCLC [26,27]. In our present study, we examined that EGFR mutation is most common and is seen in 70/188 cases (37.23%) which fall in the range of 10-66% [31,32] reported worldwide. Studies from Korea [33] Taiwan [34] Japan [35] and China [36] have observed EGFR mutation rates as 24%, 50.5%, 26.3% and 38.1% respectively. Sahoo et al., from India has reported mutation frequency in lung adenocarcinoma as 44% [37]. Regional difference has also been reported within India as 65% mutations were observed in southern population and 33% in northern population, according to Aggarwal et al [38]. Point mutation on exon 21 and in-frame deletions on exon 19 of EGFR is the most commonly observed mutation and accounts for 80-90% of all the mutations detected [17].
In our study, it could be suggested that mutations of EGFR gene were higher in elderly females than in males, which increased with increase in age in females (highest in age range of 71 – 80 yrs) and decreased with age in males (highest in age range of 51 – 60 yrs). Our observation correlated with the findings of Sahoo et al [37].
Limitations
Our retrospective study has procedural limitations due to scanty material in few small biopsies, fixation issues and assessment of staining intensity in IHC. There is a slight chance of variability in number and cross-reactivity of EGFR fusion genes studied, which may effect our observations.
In Conclusion, we discovered that males in 6th decade are more affected with lung carcinomas whereas, ALK, ROS and EGFR mutations are more common in females of 6th and 7th decades.
All ALK IHC Positive tumors were EGFR wildtype and are mutually exclusive. All ALK IHC positive tumours are ROS1 negative and vice versa. No concurrent ALK/ ROS genetic mutations were identified.
The frequency of ROS1 mutation is relatively higher compared to western counterparts. ROS1 positive tumours are associated with EGFR mutations (mainly exon 19, 20 and 21). EGFR mutations observed were in range with the previously published reports from the Asian countries and were linked with females of Asian ethnicity.
In our study all other histological variants of non-small cell lung adenocarcinoma, namely adenosquamous carcinoma, squamous carcinoma, small cell carcinoma, mucinous adenocarcinoma, sarcomatoid carcinoma, TTF-1 negative adenocarcinoma etc., were all ALK and ROS1 Negative.
Being responsive to oral tyrosine kinase inhibitors, the screening of these somatic mutations especially in females and/or never smokers could be very helpful in the further disease management of these patients.
Recommendations
We believe that Immunohistochemistry for ALK and ROS1 is an economical assay in the detection of ALK and ROS rearrangements in lung cancer, especially in elderly females. Though small proportion of NSCLCs harbor these genetic alterations, the higher incidence of ROS1 mutations compared to western counterparts, we recommend more extensive testing and screening for these mutations, to optimize diagnostic accuracy for each patient.
Statement of Transparency and Principals:
· Author declares no conflict of interest
References
- The relevance of "Nonsmoking-associated lung cancer" in India: a single-centre experience Krishnamurthy A, Vijayalakshmi R, Gadigi V, Ranganathan R, Sagar TG . Indian Journal of Cancer.2012;49(1). CrossRef
- Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer Soda M, Choi YL , Enomoto M, Takada S, Yamashita Y, Ishikawa S, Fujiwara S, et al . Nature.2007;448(7153). CrossRef
- Screening for ROS1 fusions in patients with advanced non-small cell lung carcinomas using the VENTANA ROS1 (SP384) Rabbit Monoclonal Primary Antibody Conde E, Hernandez S, Benito A, Caminoa A, Garrido P, Lopez-Rios F. Expert Review of Molecular Diagnostics.2021;21(5). CrossRef
- Management of advanced non-small cell lung cancers with known mutations or rearrangements: latest evidence and treatment approaches Shea M, Costa DB , Rangachari D. Therapeutic Advances in Respiratory Disease.2016;10(2). CrossRef
- Crizotinib in ROS1-rearranged non-small-cell lung cancer Shaw AT , Ou SI , Bang Y, Camidge DR , Solomon BJ , Salgia R, Riely GJ , et al . The New England Journal of Medicine.2014;371(21). CrossRef
- VENTANA ALK (D5F3) CDx Assay Interpretation Guide for Non-Small Cell Lung Carcinoma (NSCLC) 2015 https://www.accessdata.fda.gov/cdrh_docs/pdf14/p140025c.pdf .
- Testing for ROS1 in non-small cell lung cancer: a review with recommendations Bubendorf L, Büttner R, Al-Dayel F, Dietel M, Elmberger G, Kerr K, López-Ríos F, et al . Virchows Archiv: An International Journal of Pathology.2016;469(5). CrossRef
- MA26.07 ROS1 (SP384) immunohistochemistry inter-reader precision between 12 pathologists Hanlon Newell A, Liu W, Bubendorf L, et al . J Thorac Oncol.2018;13:S452-S453.
- Cobas EGFR Mutation Test v2, Doc. Rev. 8.0, https://pim-eservices.roche.com/eLD/api/downloads/17f87b0f-9243-eb11-0291-005056a71a5d?countryIsoCode=in .
- ROS1 rearrangements define a unique molecular class of lung cancers Bergethon K, Shaw AT , Ou SI , Katayama R, Lovly CM , McDonald NT , Massion PP , et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2012;30(8). CrossRef
- Molecular biology of anaplastic lymphoma kinase-positive anaplastic large-cell lymphoma Kutok JL , Aster JC . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2002;20(17). CrossRef
- Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer Rikova K, Guo A, Zeng Q, Possemato A, Yu J, Haack H, Nardone J, et al . Cell.2007;131(6). CrossRef
- The biology and treatment of EML4-ALK non-small cell lung cancer Sasaki T, Rodig SJ , Chirieac LR , Jänne PA . European Journal of Cancer (Oxford, England: 1990).2010;46(10). CrossRef
- KLC1-ALK: a novel fusion in lung cancer identified using a formalin-fixed paraffin-embedded tissue only Togashi Y, Soda M, Sakata S, Sugawara E, Hatano S, Asaka R, Nakajima T, Mano H, Takeuchi K. PloS One.2012;7(2). CrossRef
- EML4-ALK: honing in on a new target in non-small-cell lung cancer Horn L, Pao W. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2009;27(26). CrossRef
- Activity and safety of crizotinib in patients with ALK-positive non-small-cell lung cancer: updated results from a phase 1 study Camidge DR , Bang Y, Kwak EL , Iafrate AJ , Varella-Garcia M, Fox SB , Riely GJ , et al . The Lancet. Oncology.2012;13(10). CrossRef
- ALK rearrangements are mutually exclusive with mutations in EGFR or KRAS: an analysis of 1,683 patients with non-small cell lung cancer Gainor JF Justin F., Varghese AM , Ou SI , Kabraji S, Awad MM , Katayama R, Pawlak A, et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2013;19(15). CrossRef
- Optimizing the detection of lung cancer patients harboring anaplastic lymphoma kinase (ALK) gene rearrangements potentially suitable for ALK inhibitor treatment Camidge DR , Kono SA , Flacco A, Tan A, Doebele RC , Zhou Q, Crino L, Franklin WA , Varella-Garcia M. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2010;16(22). CrossRef
- The multifaceted roles of the receptor tyrosine kinase ROS in development and cancer Acquaviva J, Wong R, Charest A. Biochimica Et Biophysica Acta.2009;1795(1). CrossRef
- Survey of tyrosine kinase signaling reveals ROS kinase fusions in human cholangiocarcinoma Gu TL , Deng X, Huang F, Tucker M, Crosby K, Rimkunas V, Wang Y, et al . PloS One.2011;6(1). CrossRef
- Analysis of receptor tyrosine kinase ROS1-positive tumors in non-small cell lung cancer: identification of a FIG-ROS1 fusion Rimkunas WM , Crosby KE , Li D, Hu Y, Kelly ME , Gu TL , Mack JS , Silver MR , Zhou X, Haack H. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2012;18(16). CrossRef
- On the relevance of a testing algorithm for the detection of ROS1-rearranged lung adenocarcinomas Mescam-Mancini L, Lantuéjoul S, Moro-Sibilot D, Rouquette I, Souquet PJ , Audigier-Valette C, Sabourin JC , Decroisette C, Sakhri L, Brambilla E, McLeer-Florin A. Lung Cancer (Amsterdam, Netherlands).2014;83(2). CrossRef
- Clinicopathological characteristics and outcomes of ROS1-rearranged patients with lung adenocarcinoma without EGFR, KRAS mutations and ALK rearrangements Wu S, Wang J, Zhou L, Su D, Liu Y, Liang X, Zhang S, Zeng X. Thoracic Cancer.2015;6(4). CrossRef
- Targeting HER proteins in cancer therapy and the role of the non-target HER3 Hsieh AC , Moasser MM . British Journal of Cancer.2007;97(4). CrossRef
- The biology of epidermal growth factor receptor in lung cancer Scagliotti GV , Selvaggi G, Novello S, Hirsch FR . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2004;10(12 Pt 2). CrossRef
- The role of gefitinib in lung cancer treatment Giaccone G. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2004;10(12 Pt 2). CrossRef
- The role of erlotinib (Tarceva, OSI 774) in the treatment of non-small cell lung cancer Perez-Soler R. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2004;10(12 Pt 2). CrossRef
- Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib Lynch TJ , Bell DW , Sordella R, Gurubhagavatula S, Okimoto RA , Brannigan BW , Harris PL , et al . The New England Journal of Medicine.2004;350(21). CrossRef
- EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy Paez JG , Jänne PA , Lee JC , Tracy S, Greulich H, Gabriel S, Herman P, et al . Science (New York, N.Y.).2004;304(5676). CrossRef
- Kinetic analysis of epidermal growth factor receptor somatic mutant proteins shows increased sensitivity to the epidermal growth factor receptor tyrosine kinase inhibitor, erlotinib Carey KD , Garton AJ , Romero MS , Kahler J, Thomson S, Ross S, Park F, Haley JD , Gibson N, Sliwkowski MX . Cancer Research.2006;66(16). CrossRef
- Spectrum of LKB1, EGFR, and KRAS mutations in chinese lung adenocarcinomas Gao B, Sun Y, Zhang J, Ren Y, Fang R, Han X, Shen L, Liu X, Pao W, Chen H, Ji H. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2010;5(8). CrossRef
- EGFR and KRAS mutations in lung carcinomas in the Dutch population: increased EGFR mutation frequency in malignant pleural effusion of lung adenocarcinoma Smits AJJ , Kummer JA , Hinrichs JWJ , Herder GJM , Scheidel-Jacobse KC , Jiwa NM , Ruijter TEG , et al . Cellular Oncology (Dordrecht).2012;35(3). CrossRef
- EGFR and KRAS mutations in patients with adenocarcinoma of the lung Jang TW , Oak CH , Chang HK , Suo SJ , Jung MH . The Korean Journal of Internal Medicine.2009;24(1). CrossRef
- Frequent epidermal growth factor receptor gene mutations in malignant pleural effusion of lung adenocarcinoma Wu SG , Gow CH , Yu CJ , Chang YL , Yang CH , Hsu YC , Shih JY , Lee YC , Yang PC . The European Respiratory Journal.2008;32(4). CrossRef
- The Frequency of Epidermal Growth Factor Receptor Mutation of Nonsmall Cell Lung Cancer according to the Underlying Pulmonary Diseases Usui K, Ushijima T, Tanaka Y, Tanai C, Noda H, Abe N, Horiuchi H, Ishihara T. Pulmonary Medicine.2011;2011. CrossRef
- Impact of smoking status and pathologic type on epidermal growth factor receptor mutations in lung cancer Huang YS , Yang JJ , Zhang XC , Yang XN , Huang YJ , Xu CR , Zhou Q, Wang Z, Su J, Wu Y. Chinese Medical Journal.2011;124(16).
- Screening for EGFR mutations in lung cancer, a report from India Sahoo R, Harini VV , Babu VC , Patil Okaly GV , Rao S, Nargund A, Venkataswamy E, Rao R, Kumar BSA . Lung Cancer (Amsterdam, Netherlands).2011;73(3). CrossRef
- A study of EGFR mutation in nonsmoker NSCLC: Striking disparity between north and south India patients. | Request PDF Aggarwal S, Patil S, Minhans S, Pungliya M, Soumitra N. J Clin Oncol.2012;2012(30(Suppl)):E18041. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details