Evaluation of Tumour Budding in Head and Neck Squamous Cell Carcinoma and Its Relationship with other Histological Parameters of Prognosis
Download
Abstract
Background: Tumor budding (TB) is an emerging and promising histopathological prognostic parameter in oral squamous cell carcinoma (SCC). Tumour budding shows positive correlation with lymph node metastasis, higher pattern of invasion, high grade of tumor and more depth of invasion. These histopathological parameters are associated with poor prognosis, high rate of recurrence and these prognostic factors can be routinely used by clinicians for planning of multimodality treatment.
Method: The present study was a cross-sectional study carried out on total of 239 patients with oral squamous cell carcinoma who attended the surgical oncology unit of Kalyan Singh Super Speciality Cancer Institute, Lucknow, Uttar Pradesh, India, from November 2020 to June 2022. Descriptive statistical analysis has been carried out in the present study. We used Fischer Exact Probability test and chi-square test analysis in order to identify relationship between different histological features, significance was considered when p<0.05.
Results: Majority of study population (69.87%) were in the age group of less than 54 years of age. Age group (<54years) was significantly associated with tumor budding (p<0.05). Cases diagnosed as moderately differentiated SCC were 68.6 % and well differentiated SCC were 25.1%. 74 cases were associated with high intensity of tumor budding and 59 cases with low intensity of tumor budding (p<0.05). Most common worst pattern of invasion was type 4 (61.9%) followed by type 5 (26.4%), strong statistical association was observed with intensity of tumor budding (p<0.05). Most of the cases exhibited maximum depth of invasion (DOI) >1cm (100 cases). DOI was significantly associated with intensity of tumor budding (p<0.05). Out of 102 involved lymph node cases, 91 cases were associated with tumor budding 55 cases of these associated with high intensity tumor budding. Tumor budding was also significantly associated with lymph node metastasis (p<0.05).
Conclusion: Present study showed a strong correlation of tumor budding with lymph node metastasis, depth of invasion and worst pattern of invasion. Thus, this study emphasizes the importance of tumor budding evaluation in routine pathology reporting and similar to depth of invasion TB should be included in AJCC TNM staging.
Introduction
Squamous cell carcinoma (SCC) is the most common malignancy of oral cavity, it accounts for more than 90% of oral cavity cancers. It has been reported that 20-40% cases of oral squamous cell carcinoma have occult metastasis during presentation to hospital [1,2].
Lymph node negative cases show better prognosis compared to cases with lymph nodes metastasis. Hence, lymph node involvement is very important prognosticator in oral SCC cases [3]. Therefore, it is imperative that histopathological parameters should be identified that are associated with possible lymph node metastasis. These prognostic indicators help to stratify patients for surgical treatment including neck dissection, radiotherapy and chemotherapy.
Tumor budding (TB) is an emerging and promising histopathological prognostic parameter in oral SCC. In the invasive tumor front, a separate single or cluster of tumor cells is known as tumor budding [4]. Tumour budding shows positive correlation with lymph node metastasis, higher pattern of invasion, high grade of tumor and more depth of invasion. These histopathological parameters are associated with poor prognosis and high rate of recurrence. Study done by Haddad TS et al and a separate study done by Kucuk S et al proved prognostic role of TB for colorectal and stomach carcinoma but for oral squamous cell carcinoma it’s prognostic role is under evaluation [5,6]. Due to paucity of literature in support of prognostic role of tumor budding and worst pattern of invasion, College of American Pathologist guideline has not incorporated tumour budding as an essential criteria for reporting [7,8] in oral SCC.
In the present study we have attempted to highlight the importance of tumor budding as a risk factor for lymph node metastasis and its relationship with worst pattern of invasion in oral squamous cell carcinoma. These prognostic factors can be routinely used by clinicians for planning of multimodality treatment [9, 10].
Materials and Methods
The present study was a cross-sectional study carried out on total of 239 patients with oral squamous cell carcinoma who attended the surgical oncology unit of Kalyan Singh Super Speciality Cancer Institute, Lucknow, Uttar Pradesh, India, from November 2020 to June 2022 after considering inclusion and exclusion criteria. The inclusion criteria for our study were histologically confirmed primary squamous cell carcinoma without any previous history of chemotherapy or radiotherapy and who were eligible for curative radical surgery. Patients who were eligible for palliative surgery with unresectable or metastatic tumors and incomplete medical records were excluded.
Neck dissection was performed in all patients with clinico-radiologically/ FNAC proved positive nodes as well as in patients with large tumors and clinically negative nodes. Surgery was followed by radiotherapy with or without chemotherapy depending on histopathology report and the national guidelines. Finally, patient entered a follow-up program which included clinical and radiological assessments.
All specimens were fixed in 10% neutral buffered formalin and were grossed according to American Joint Committee on Cancers (AJCC) protocol. Tissue was processed in fully automated tissue processor subsequently embalmed in paraffin blokes and cut at 3-5 µm, H&E stained sections were prepared for histopathological analysis. Reporting was done according to College of American Pathologist (CAP) guideline and AJCC 8th edition. Based on Almangush et al scoring criteria, tumor buds were evaluated [11]. The presence of a single tumor cell or small cluster of tumor cells (<5) at the invasive front is defined as tumor bud. Slides were scanned “using 4× microscope” objective to select areas with highest tumor budding then TB was counted at higher magnification (20×). Tumor budding was graded as low intensity (LI) and high intensity (HI). The cut off value for low intensity was <5 buds per field (magnification 20x) and cut off value for high intensity was > or = 5 buds per field (magnification 20x). Cases with no tumor budding on H&E section were revaluated in pancytokeratin stained sections. To avoid bias, each section was evaluated by two observers and then jointly by consensus. Based on previous literature worst pattern of invasion (WPOI) was determined [12,13]. In type 1 pattern tumor invades stroma in broad pushing manner, in type 2 pattern tumor invades stroma in solid cords and strands (finger-like), type 3 pattern presents as invasive islands of less than 15 tumor cells clusters, type 4 pattern presents as invasive islands of more than15 tumor cells clusters, type 5 pattern shows tumor islands more than 1 mm away from the progressive end of the tumor or presence of extra tumoral perineural invasion. As per CAP guidelines depth of invasion (DOI) was measured from the basement membrane of adjacent normal mucosa to the deepest point of invasion of the tumor.
Statical analysis
Descriptive statistical analysis has been carried out in the present study. We used Fischer Exact Probability test and chi-square test analysis in order to identify relations between different histological features, significance was considered when p<0.05. The statistical software namely Open Epi Version 3 have been used for the analysis of the data and Microsoft word and Excel have been used to generate tables.
Aims and Objectives
This study aims to evaluate the significance of the tumor budding as a risk factor for lymph node metastasis and its relationship with worst pattern of invasion, depth of invasion and other clinicopathological parameters in oral squamous cell carcinoma patients.
Results
A total of 239 were cases included in this study, who met the eligibility criteria. Majority of study population (69.87%) were in the age group of less than 54 years (Table 1).
| Tumor budding grade | |||||
| Gender | A | HI | LI | Grand Total | p Value |
| Female | 8 | 26 | 15 | 49 | p < .05 |
| Male | 60 | 65 | 65 | 190 | |
| Grand Total | 68 | 91 | 80 | 239 | |
| Diagnosis | Tumor budding grade | ||||
| A | HI | LI | Grand Total | p Value | |
| Well differentiated SCC | 35 | 7 | 18 | 60 | |
| Moderately differentiated SCC | 31 | 74 | 59 | 164 | p < .05. |
| Poorly differentiated SCC | 2 | 10 | 3 | 15 | |
| Grand Total | 68 | 91 | 80 | 239 | |
| Lymph nodes (Positive/ Negative for metastasis) | Tumor budding grade | ||||
| A | HI | LI | Grand Total | p Value | |
| Negative | 57 | 36 | 44 | 137 | p < .05. |
| Positive | 11 | 55 | 36 | 102 | |
| Grand Total | 68 | 91 | 80 | 239 | |
| Depth of invasion | Tumor budding grade | ||||
| A | HI | LI | Grand Total | p Value | |
| < 0.5 cm | 21 | 5 | 5 | 31 | p < .05. |
| 0.5 cm - 1.0 cm | 26 | 30 | 36 | 92 | |
| > 1.0 cm | 16 | 50 | 34 | 100 | |
| Could not be measured | 5 | 6 | 5 | 16 | |
| Grand Total | 68 | 91 | 80 | 239 | |
| Worst pattern | Tumor budding grade | ||||
| A | HI | LI | Grand Total | p Value | |
| Type2 | 4 | 0 | 0 | 4 | |
| Type 3 | 22 | 1 | 5 | 28 | p < .05. |
| Type 4 | 37 | 57 | 54 | 148 | |
| Type 5 | 5 | 33 | 21 | 59 | |
| Grand Total | 68 | 91 | 80 | 239 | |
| N staging | Tumor budding grade | ||||
| A | HI | LI | Grand Total | p Value | |
| pN0 | 58 | 31 | 42 | 131 | |
| pN1 | 2 | 16 | 15 | 33 | |
| pN2a | 1 | 4 | 2 | 7 | |
| pN2b | 3 | 16 | 10 | 29 | |
| pN2c | 1 | 2 | 3 | ||
| pN3b | 3 | 20 | 9 | 32 | |
| Grand Total | 68 | 91 | 80 | 235 | |
| P staging | Tumor budding grade | ||||
| A | HI | LI | Grand Total | p Value | |
| pT1 | 21 | 5 | 6 | 32 | p < .05. |
| pT2 | 22 | 24 | 35 | 81 | |
| pT3 | 7 | 36 | 23 | 66 | |
| pT4a | 18 | 25 | 15 | 58 | |
| pT4b | 1 | 1 | 2 | ||
| Grand Total | 68 | 91 | 80 | 239 |
A, Absent; LI, Low intensity; HI, High intensity; SCC, Squamous cell carcinoma
Age group (<54years) was significantly associated with tumor budding (p<0.05). 79.4% of the study subjects comprised of males and 20.50% females with a male to female ratio of 3.8:1 (Table 1). The buccal mucosa was the most common site of tumor comprising of 129 cases (53.9 % of the cases), followed by tongue 63 cases (26.3 % of the cases) (Table 1). 164 (68.6%) cases were diagnosed as moderately differentiated SCC followed by 60 cases of well differentiated SCC (25.1%) (Table 1). Out of these 164 cases, 74 cases were associated with high intensity of tumor budding and 59 cases with low intensity of tumor budding (Table 1) and Figures 1, 2, 3 and 4.
Figure 1. Photomicrograph Showing High Intensity Tumor Budding in OSCC (H and E 20X).
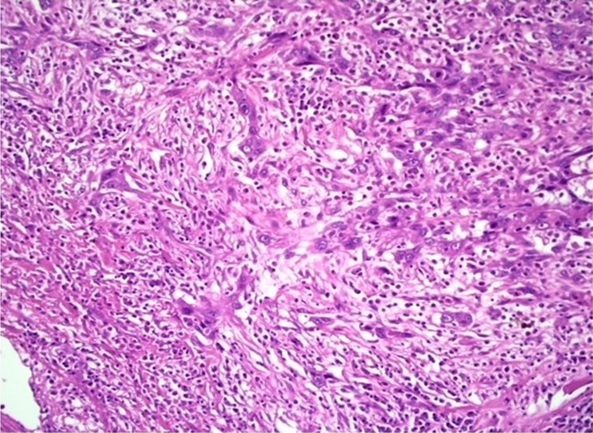
Figure 2. Photomicrograph Showing High Intensity Tumor Budding in OSCC (H and E 10X).
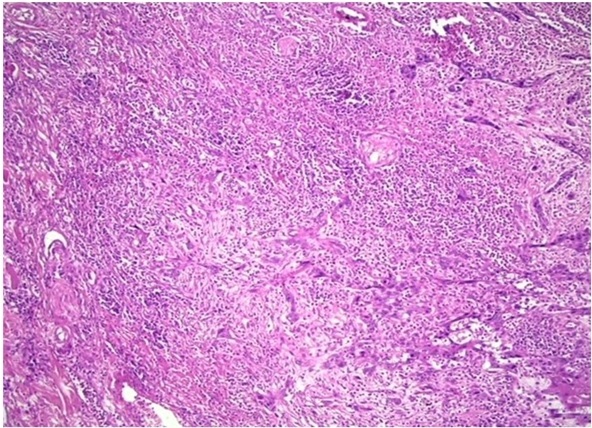
Figure 3. Photomicrograph Showing Low Intensity Tumor Budding in OSCC (H and E 20X).
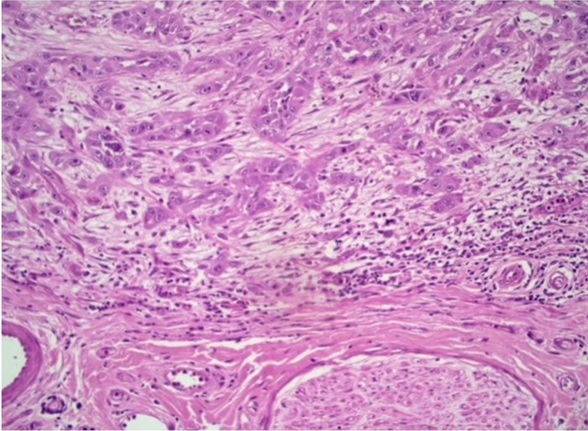
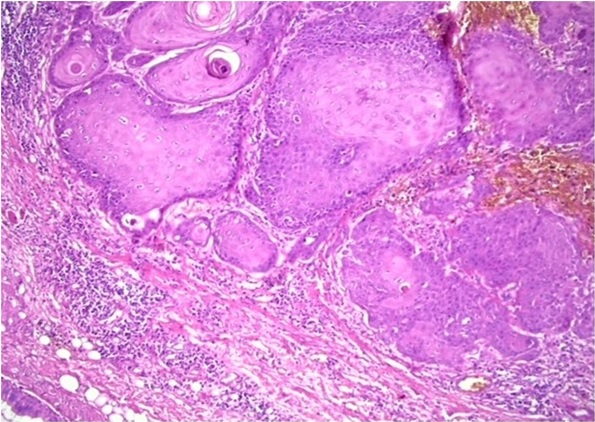
Figure 4. Photomicrograph Showing Absence of Intensity Tumor Budding in OSCC (H and E 20X).
Degree of differentiation was significantly associated with intensity of tumor budding (p<0.05). Most common worst pattern of invasion was type 4 (61.9%) followed by type 5 (26.4%) (Table 1). As expected, strong statical association was observed between worst pattern of invasion and intensity of tumor budding (p<0.05). Most of the cases exhibited maximum depth of invasion >1cm (100 cases) followed by 0.5-1cm (92 cases). Out of these 100 cases, 50 cases were associated with high intensity of tumor budding and 34 cases low intensity of tumor budding (Table 1). Depth of invasion was significantly associated with intensity of tumor budding (p<0.05). Ipsilateral/ bilateral neck dissection was done in 239 cases. Of these, 102 cases (42.6%) had histologically involved lymph nodes, while rest of the 137 cases (57.3%) had histologically uninvolved lymph nodes. Out of 102 involved lymph node cases, 91 cases were associated with tumor budding 55 cases of these associated with high intensity tumor budding. Tumor budding was also significantly associated with lymph node metastasis (p<0.05).
Discussion
Tumour budding was first identified by Gabbert et al. at the invasive front in colorectal cancers [14,15]. However, it was Morodomi et al. and Hase et al, who gave the terminology of “budding” because they observed that these undifferentiated cells and nests appeared to be budding out from larger tumor islands [16,17].
Tumor budding (TB) is a histopathological feature characterized by presence of separated single or cluster of tumor cells in the invasive tumor front within the stroma. During the process of tumor budding, cancer cells lose cell adhesion, resist apoptosis and by causing peritumoral stromal degradation tumor cells invade deep [18]. TB characterizes the aggressiveness and invasive nature of tumor [19].
Tumor budding as an indicator of prognosis is simple and reproducible, in addition, it can be evaluated with conventional H&E staining with no need for expensive tools.
The results of present study showed that tumor budding had a significant relationship with clinicopathological parameters such as lymph node metastasis, worst pattern of invasion, depth of invasion, histopathological grading of tumor along with demographic factors of gender and age.
Tumor budding is now recognized as an adverse prognostic factor in different epithelial tumors, including head and neck squamous cell carcinoma. The most recent reviews about head and neck cancer reported that high tumor budding values were significantly associated with invasion, nodal metastasis and worse prognosis [20]. Mäkitie et al highlighted not only the prognostic significance of tumor budding in head and neck carcinoma but also its potential for improving clinical decision making in terms of recommending optimal individualized treatment [21]. This study showed a strong statistical correlation between the presence of tumor buds/ number of buds and LN metastasis, which is in harmony with results of previous studies done by Lu W et al and another study done by Togni L et al [22,23]. Study done by Seki M,et al and by Dirven R et al showed presence of TB in low-stage oral squamous cell carcinoma, where TB has been significantly associated with lymph node metastasis and poor prognosis [24,25]. This suggests similar to depth of invasion TB should be included in AJCC TNM staging and in treatment decision-making.
The most common worst pattern of invasion that observed was type 4 (61.92%) followed by type 5 (24.68%) and were significantly associated with TB. In this study majority of cases with metastatic lymph node associated with type 4 and type 5 pattern. Invasive patterns with dyscohesive cells (type 4, type 5) that penetrates deep into stroma and undergo epithelial mesenchymal transition (EMT) for migration [26]. This EMT helps in tumor bud formation and metastasis [27]. Wang et al. suggested that TB may represent cells undergoing EMT as they showed diminshed E cadherin expression and overexpression of vimentin in tongue squamous cell carcinoma. In addition, Jensen et al. have demonstrated enhanced expression of ZEB1 and PPRX1 genes, both renowned EMT stimulators in tumor buds using RNA sequencing. Thus tumour budding can be considered as a histological marker of EMT [28,29].
In our study most of the cases exhibited maximum depth of invasion >1cm (100 cases). Out of these 100 cases, 84 cases showed tumor budding (HI-50 cases, LI-34 cases). Depth of invasion was significantly associated with TB, which is in harmony with results of the study done by Wahab A et al [30]. Increased depth of invasion by cancer cells induces molecular events that helps in production of isolated tumor cells or tumor buds [31]. Depth of invasion is a prognosticator of oral squamous cell carcinoma. It has been found that increased risk of recurrence and lymph node metastasis is significantly associated with more than 1cm of depth of invasion [32,33].
A total of 102 cases were (42.6%) associated with histologically involved lymph node and most of the cases with involved lymph node reported as pN1 stage (33 cases) followed by N3b (32cases).
Out of these 33 cases of pN1, 31 cases were associated with TB and 32 cases of pN3b, and 29 cases of N2b were associated with TB. In this study tumor budding was significantly associated with pN staging. Literature search showed that tumor budding is an important risk factor for predicting LN metastasis in all stages of OSCC and is associated with a poorer prognosis even in early-stage tumors [34,35]. Majority of the study population was in the age group of less than 54 years (69.87%). OSCC was more common in younger age group and there was significant association with tumor budding. OSCC in younger population demonstrated high intensity of tumor budding. Other than tobacco and alcohol consumption, frequent risk factors for OSSC in younger age group considered are dietary/nutritional deficiency, genetic predisposition and exposure to high-risk HPV types [36,37].
In the present study incidence of oral squamous cell carcinoma was higher among males than females (3.8:1) and was significantly associated with tumor budding.
In our study most of the cases were histologically diagnosed as moderately differentiated SCC (68.61%) followed by well differentiated (25.1%). It was observed that a high intensity of tumor budding showed a significant correlation with histological grade of tumor.
In conclusion, present study showed a strong correlation of tumor budding with lymph node metastasis, depth of invasion and worst pattern of invasion. These histopathological features have prognostic and predictive significance, which can routinely be used for planning of multimodality treatment including radiotherapy and chemotherapy. Thus, this study emphasizes the importance of tumor budding evaluation in routine pathology reporting and similar to depth of invasion TB should be included in AJCC TNM staging.
Acknowledgments
Statement of Transparency and Principals:
· Author declares no conflict of interest
· Study was approved by Research Ethic Committee of author affiliated Institute.
· Study’s data is available upon a reasonable request.
· All authors have contributed to implementation of this research.
References
- The global incidence of lip, oral cavity, and pharyngeal cancers by subsite in 2012 Shield KD , Ferlay J, Jemal A, Sankaranarayanan R, Chaturvedi AK , Bray F, Soerjomataram I. CA: a cancer journal for clinicians.2017;67(1). CrossRef
- The influence of lymph node metastasis in the treatment of squamous cell carcinoma of the oral cavity, oropharynx, larynx, and hypopharynx: N0 versus N+ Layland MK , Sessions DG , Lenox J. The Laryngoscope.2005;115(4). CrossRef
- Cervical lymph node metastasis: assessment of radiologic criteria Brekel MW , Stel HV , Castelijns JA , Nauta JJ , Waal I, Valk J, Meyer CJ , Snow GB . Radiology.1990;177(2). CrossRef
- Tumor budding is associated with poor prognosis of oral squamous cell carcinoma and histologically represents an epithelial-mesenchymal transition process Hong K, Oh K, Shin W, Yoon H, Lee J, Hong S. Human Pathology.2018;80. CrossRef
- Improving tumor budding reporting in colorectal cancer: a Delphi consensus study Haddad TS , Lugli A, Aherne S, Barresi V, Terris B, Bokhorst J, Brockmoeller SF , et al . Virchows Archiv: An International Journal of Pathology.2021;479(3). CrossRef
- Prognostic value of tumour budding in stomach cancers Kucuk S. International Journal of Clinical Practice.2021;75(12). CrossRef
- Development of a New Outcome Prediction Model in Early-stage Squamous Cell Carcinoma of the Oral Cavity Based on Histopathologic Parameters With Multivariate Analysis: The Aditi-Nuzhat Lymph-node Prediction Score (ANLPS) System Arora A, Husain N, Bansal A, Neyaz A, Jaiswal R, Jain K, Chaturvedi A, Anand N, Malhotra K, Shukla S. The American Journal of Surgical Pathology.2017;41(7). CrossRef
- Clinicopathological correlation of tumor-stroma ratio and inflammatory cell infiltrate with tumor grade and lymph node metastasis in squamous cell carcinoma of buccal mucosa and tongue in 41 cases with review of literature Rani P, Gupta AJ , Mehrol C, Singh M, Khurana N, Passey JC . Journal of Cancer Research and Therapeutics.2020;16(3). CrossRef
- CD56-positive lymphocyte infiltration in relation to human papillomavirus association and prognostic significance in oropharyngeal squamous cell carcinoma Wagner S, Wittekindt C, Reuschenbach M, Hennig B, Thevarajah M, Würdemann N, Prigge E, Knebel Doeberitz M, Dreyer T, Gattenlöhner S, Klussmann JP . International Journal of Cancer.2016;138(9). CrossRef
- The impact of worst pattern of invasion on the extension of surgical margins in oral squamous cell carcinoma Köhler HF , Vartanian JG , Pinto CAL , Silva Rodrigues LFP , Kowalski LP . Head & Neck.2022;44(3). CrossRef
- A simple novel prognostic model for early stage oral tongue cancer Almangush A, Coletta RD , Bello IO , Bitu C, Keski-Säntti H, Mäkinen LK , Kauppila JH , et al . International Journal of Oral and Maxillofacial Surgery.2015;44(2). CrossRef
- Worst pattern of invasion in oral squamous cell carcinoma is an independent prognostic factor Mishra A, Das A, Dhal I, Shankar R, Bhavya BM , Singh N, Tripathi P, et al . Journal of Oral Biology and Craniofacial Research.2022;12(6). CrossRef
- A semi-quantitative World Health Organization grading scheme evaluating worst tumor differentiation predicts disease-free survival in oral squamous carcinoma patients Jain D, Tikku G, Bhadana P, Dravid C, Grover RK . Annals of Diagnostic Pathology.2017;29. CrossRef
- Tumor Budding as a Strong Prognostic Indicator in Invasive Ampullary Adenocarcinomas Ohike N, Coban I, Kim GE , Basturk O, Tajiri T, Krasinskas A, Bandyopadhyay S, et al . The American journal of surgical pathology.2010;34(10). CrossRef
- Prognostic value of immunohistochemical analysis of tumor budding in colorectal carcinoma Ohtsuki K, Koyama F, Tamura T, Enomoto Y, Fujii H, Mukogawa T, Nakagawa T, et al . Anticancer Research.2008;28(3B).
- Construction of a pathological risk model of occult lymph node metastases for prognostication by semi-automated image analysis of tumor budding in early-stage oral squamous cell carcinoma Pedersen NJ , Jensen DH , Lelkaitis G, Kiss K, Charabi B, Specht L, Buchwald C. Oncotarget.2017;8(11). CrossRef
- Tumor Budding: The Name is EMT. Partial EMT Grigore AD , Jolly MK , Jia D, Farach-Carson MC , Levine H. Journal of Clinical Medicine.2016;5(5). CrossRef
- Tumor microenvironment and noncoding RNAs as co-drivers of epithelial-mesenchymal transition and cancer metastasis Drak Alsibai K, Meseure D. Developmental Dynamics: An Official Publication of the American Association of Anatomists.2018;247(3). CrossRef
- Tumour 'budding' as an index to estimate the potential of aggressiveness in rectal cancer Ueno H, Murphy J, Jass JR , Mochizuki H, Talbot IC . Histopathology.2002;40(2). CrossRef
- Tumor budding to investigate local invasion, metastasis and prognosis in temporal bone squamous cell carcinoma Alessandrini L, Zanoletti E, Cazzador D, Sbaraglia M, Franz L, Tealdo G, Frigo AC , et al . Pathology, Research and Practice.2022;229. CrossRef
- Hallmarks of cancer: Tumor budding as a sign of invasion and metastasis in head and neck cancer Mäkitie AA , Almangush A, Rodrigo JP , Ferlito A, Leivo I. Head & Neck.2019;41(10). CrossRef
- Epithelial-Mesenchymal Plasticity in Cancer Progression and Metastasis Lu W, Kang Y. Developmental Cell.2019;49(3). CrossRef
- The Emerging Impact of Tumor Budding in Oral Squamous Cell Carcinoma: Main Issues and Clinical Relevance of a New Prognostic Marker Togni L, Caponio VCA , Zerman N, Troiano G, Zhurakivska K, Lo Muzio L, Balercia A, Mascitti M, Santarelli A. Cancers.2022;14(15). CrossRef
- Tumour budding evaluated in biopsy specimens is a useful predictor of prognosis in patients with cN0 early stage oral squamous cell carcinoma Seki M, Sano T, Yokoo S, Oyama T. Histopathology.2017;70(6). CrossRef
- Tumor thickness versus depth of invasion - Analysis of the 8th edition American Joint Committee on Cancer Staging for oral cancer Dirven R, Ebrahimi A, Moeckelmann N, Palme CE Carsten Erich, Gupta R, Clark J. Oral Oncology.2017;74. CrossRef
- The prognostic role of histologic grade, worst pattern of invasion, and tumor budding in early oral tongue squamous cell carcinoma: a comparative study Xu B, Salama AM , Valero C, Yuan A, Khimraj A, Saliba M, Zanoni DK , Ganly I, Patel SG , Katabi N, Ghossein R. Virchows Archiv: An International Journal of Pathology.2021;479(3). CrossRef
- Tumor budding - A promising prognostic histopathological parameter in oral squamous cell carcinoma - A comparative immunohistochemical study Joshi P, Pol J, Chougule M, Jadhav K, Patil S, Patil S. Journal of oral and maxillofacial pathology: JOMFP.2020;24(3). CrossRef
- Tumor budding correlates with poor prognosis and epithelial-mesenchymal transition in tongue squamous cell carcinoma Wang C, Huang H, Huang Z, Wang A, Chen X, Huang L, Zhou X, Liu X. Journal of Oral Pathology & Medicine: Official Publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology.2011;40(7). CrossRef
- Molecular profiling of tumour budding implicates TGFβ-mediated epithelial-mesenchymal transition as a therapeutic target in oral squamous cell carcinoma Jensen DH , Dabelsteen E, Specht L, Fiehn AMK , Therkildsen MH , Jønson L, Vikesaa J, Nielsen FC , Buchwald C. The Journal of Pathology.2015;236(4). CrossRef
- The budding and depth of invasion model in oral cancer: A systematic review and meta-analysis Wahab A, Onkamo O, Pirinen M, Almangush A, Salo T. Oral diseases.2022;28(2). CrossRef
- MAML1 and TWIST1 co-overexpression promote invasion of head and neck squamous cell carcinoma Ardalan Khales S, Ebrahimi E, Jahanzad E, Ardalan Khales S, Forghanifard MM . Asia-Pacific Journal of Clinical Oncology.2018;14(5). CrossRef
- Histological assessment of budding and depth of invasion (BD) model in biopsies of oral squamous cell carcinoma Acharya S, Raj M, Hallikeri K, Desai A. Journal of oral and maxillofacial pathology: JOMFP.2020;24(3). CrossRef
- Is the Depth of Invasion a Marker for Elective Neck Dissection in Early Oral Squamous Cell Carcinoma? Aaboubout Y, Toom QM , Ridder MAJ , De Herdt MJ , Steen B, Lanschot CGF , Barroso EM , et al . Frontiers in Oncology.2021;11. CrossRef
- Tumor budding is an independent prognostic marker in early stage oral squamous cell carcinoma: With special reference to the mode of invasion and worst pattern of invasion Shimizu S, Miyazaki A, Sonoda T, Koike K, Ogi K, Kobayashi J, Kaneko T, et al . PloS One.2018;13(4). CrossRef
- Tumor Budding and Worse Pattern of Invasion Can Predict Nodal Metastasis in Oral Cancers and Associated With Poor Survival in Early-Stage Tumors Chatterjee D, Bansal V, Malik V, Bhagat R, Punia RS , Handa U, Gupta A, Dass A. Ear, Nose, & Throat Journal.2019;98(7). CrossRef
- Clinicopathologic Characteristics of Young Patients with Oral Squamous Cell Carcinoma Mneimneh WS , Xu B, Ghossein C, Alzumaili B, Sethi S, Ganly I, Khimraj A, Dogan S, Katabi N. Head and Neck Pathology.2021;15(4). CrossRef
- High-risk human papillomavirus in oral squamous cell carcinoma of young patients Kaminagakura E, Villa LL , Andreoli MA , Sobrinho JS , Vartanian JG , Soares FA , Nishimoto IN , Rocha R, Kowalski LP . International Journal of Cancer.2012;130(8). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details