Esophageal Pleural Fistula in Esophageal Squamous Cell Carcinoma: A Diagnostic Challenge
Download
Abstract
Esophageal pleural fistula (EPF) is a rare but serious complication of esophageal squamous cell carcinoma (ESCC), characterized by an abnormal communication between the esophagus and the pleural space. This abstract provides an overview of EPF in the context of ESCC, focusing on its etiology, clinical presentation, diagnosis, and treatment options. ESCC is a prevalent malignancy worldwide, particularly in regions with a high incidence of tobacco and alcohol consumption. As the tumor progresses, it can invade adjacent structures, including the pleura. The infiltration of the tumor into the pleural space may lead to the formation of an EPF. Patients with EPF often present with symptoms such as cough, dyspnea, chest pain, recurrent pneumonia, or subcutaneous emphysema. However, the clinical manifestations can vary widely, making the diagnosis challenging. Radiological imaging, including contrast-enhanced computed tomography (CT) scans and barium studies, plays a crucial role in detecting the presence and extent of the fistula. The management of EPF in ESCC involves a multidisciplinary approach. Treatment options depend on various factors, including the patient’s overall health status, tumor stage, and extent of the fistula. Conservative measures, such as nutritional support, antibiotics, and drainage of pleural effusion, may be employed in patients who are not surgical candidates. However, the definitive treatment for EPF is usually surgical intervention, which aims to resect the involved segment of the esophagus and repair the fistula. In some cases, palliative strategies, such as stenting or endoscopic interventions, may be considered to alleviate symptoms and improve quality of life. Through this article, we debate on a 47-year-old man presenting with esophageal fistula as the complication of esophageal squamous cell carcinoma. He has always been concerning about dysphagia, however endoscopic biopsies have been unsatisfying till now.
Introduction
Esophageal Esophageal carcinoma is the seventh most frequent cancer and the sixth cancer that ends to death [1]. There are two forms of esophageal cancers including SCC and adenocarcinoma [2]. SCC mostly affect the third part of esophagus and the most common presentation is stenosis [1]. Esophageal pleural fistula (EPF) is a rare yet potentially life-threatening complication that can arise in patients with esophageal squamous cell carcinoma (ESCC). It is characterized by an abnormal connection between the esophagus and the pleural space, leading to the leakage of gastrointestinal contents into the thoracic cavity. EPF poses significant diagnostic and therapeutic challenges due to its complex nature and limited treatment options [3,4]. This introduction provides an overview of EPF in the context of ESCC, highlighting its etiology, clinical presentation, diagnosis, and available treatment strategies. ESCC is a prevalent form of esophageal cancer worldwide, particularly in regions with high rates of tobacco and alcohol consumption [5]. The development of EPF often occurs as ESCC progresses and infiltrates surrounding structures, such as the pleura, resulting in fistula formation. Several factors contribute to the occurrence of EPF, including tumor invasion, necrosis, and local tissue inflammation [6]. The clinical presentation of EPF varies and can include symptoms such as cough, dyspnea, chest pain, recurrent pneumonia, and subcutaneous emphysema [7]. However, these symptoms are nonspecific and may overlap with other thoracic conditions, making accurate diagnosis challenging. Radiological imaging plays a critical role in detecting and evaluating EPF. Contrast-enhanced computed tomography (CT) scans and barium studies can provide valuable information regarding the presence and extent of the fistula [8]. Managing EPF in patients with ESCC requires a multidisciplinary approach. Treatment options depend on various factors, including the patient’s overall health status, tumor stage, and the extent of the fistula. Conservative measures, such as nutritional support, antibiotics, and drainage of pleural effusion, may be utilized in cases where surgical intervention is not feasible [9]. However, surgical resection remains the preferred approach for definitive management of EPF. The surgical procedure aims to resect the involved segment of the esophagus and repair the fistula. In some cases, palliative interventions, such as stenting or endoscopic techniques, may be considered to alleviate symptoms and improve quality of life [10]. Despite advancements in diagnosis and treatment, EPF due to ESCC continues to present significant challenges in clinical practice. Improved understanding of its underlying mechanisms, early recognition, accurate diagnosis, and the development of novel therapeutic approaches are critical areas for further research. Esophageal fistula count as one of the serious complications of this rapidly progressive cancer [3] esophageal fistula predict poorer outcome and lower quality of life [2]. Incidence of malignant esophageal fistula is about 13%, Treatment modalities deviate from palliation to active and, if possible, radical surgical interventions [6].
Case report
A 47-year-old male presented to the hospital with a complaint of dysphagia that had been persistent for a month. Initial upper endoscopy revealed thick, white exudate on the mucosa of the lower part of the esophagus (Figure 1).
Figure 1. Initial Upper Endoscopy Revealed thick, white Exudate on the Mucosa of the Lower Part of the Esophagus. Histopathologic evaluation of the specimens from this part of esophagus confirmed the diagnosis of esophageal candidiasis.
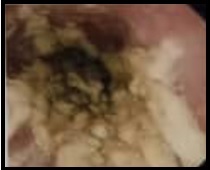
Biopsy results indicated acute esophagitis and candidiasis, but no signs of malignancy. A spiral abdominopelvic CT scan was performed, which showed wall thickening and heterogeneous enhancement in the distal esophagus. The patient received treatment for candidiasis, and a follow-up endoscopy was conducted two months later. Luminal narrowing was observed in the third segment of the esophagus during this procedure, and biopsies were taken from the distal part of the esophagus. Pathology once again revealed esophagitis but no signs of neoplasia. Dysphagia continued to be the primary complaint. Another endoscopy was performed one month later, revealing luminal narrowing and irregularity in the distal esophagus preventing from advancement of scope into the stomach (Figure 2).
Figure 2. Follow-up Endoscopy Revealed Luminal Narrowing and Irregularity in the Distal Esophagus Preventing from Advancement of Scope into the Stomach.

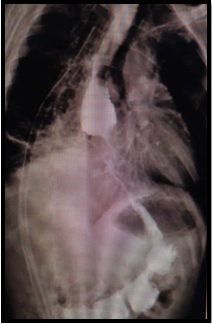
Multiple biopsies were taken, all of which showed no evidence of malignancy. A barium swallow study was conducted, revealing shoulder and mucosal irregularity in distal third of esophagus and a small contrast collection in the right hemithorax, suggesting the presence of a fistula (Figure 3).
Figure 3. A barium Swallow Study was Conducted, Revealing Shoulder and Mucosal Irregularity in Distal third of Esophagus and a Small Contrast Collection in the Right Hemithorax, Suggesting the Presence of a Fistula.
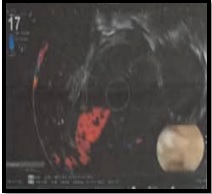
Abdominal ultrasound detected fluid in both hemithoraxes, with loculated empyema observed in the right pleural space. Further evaluation through endoscopic ultrasound (EUS) showed a circular ill-defined hypoechoic lesion originating from the second and third layers of the esophagus invading the serosa. The passage of tumor from serosa was evident. No significant mediastinal lymph node was detected indicative of at least T4N0Mx stage of tumor (Figure 4).
Figure 4. Endoscopic Ultrasound (EUS) Showed a Circular Ill-defined Hypoechoic Lesion Originating from the Second and third Layers of the Esophagus Invading the Serosa. The passage of tumor from serosa was evident. No significant mediastinal lymph node was detected indicative of at least T4N0Mx stage of tumor.
Fine-needle aspiration (FNA) was attempted but did not yield sufficient sample. The patient was subsequently referred to a surgeon, who performed an esophagectomy. During the surgery, it was discovered that the tumor had adhered to the inferior lobe of the right lung, and there was evidence of abscess formation in that area. Esophagectomy, wedge resection of the lung, and abscess removal were carried out. Pathological evaluation confirmed a well-differentiated (G1) squamous cell carcinoma (SCC) measuring 9 x 4.5 x 2.5 cm confined to the tubular esophagus, without involvement of the esophagogastric junction. The surgical margins were not involved, and no lymphovascular invasion or regional lymph node involvement was detected. The lung mass was identified as SCC originating from the esophagus, with associated abscess formation.
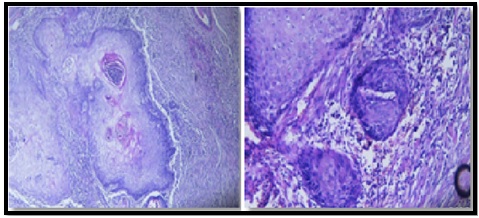
The histopathological examination of the specimen obtained from the esophageal pleural fistula due to ESCC revealed significant findings. Microscopic evaluation showed that the tumor was primarily located within the tubular esophagus and did not involve the esophagogastric junction. The size of the tumor was measured to be 9 x 4.5 x 2.5 cm, and it was identified as squamous cell carcinoma histologically. The tumor was classified as G1, indicating a well-differentiated grade. Importantly, the examination indicated that there was no involvement of the proximal and distal margins, lymphovascular invasion was not detected, and no regional lymph node invasion was present (T4NX). Additionally, the histopathology report noted the presence of abscess formation in the lung, which was determined to be involved by the esophageal SCC. These findings provide crucial information about the nature, stage, and characteristics of the esophageal pleural fistula associated with ESCC (Figure 5).
Figure 5. Microscopic Evaluation Showed that the Ttumor was Primarily Located within the Tubular Esophagus and did not Involve the Esophagogastric Junction. The size of the tumor was measured to be 9 x 4.5 x 2.5 cm, and it was identified as squamous cell carcinoma histologically. The tumor was classified as G1, indicating a well-differentiated grade. Importantly, the examination indicated that there was no involvement of the proximal and distal margins, lymphovascular invasion was not detected, and no regional lymph node invasion was present (T4NX).
Discussion
Esophageal cancer is the seventh most common type of cancer and ranks as the sixth leading cause of cancer-related death [11]. It comprises two main forms: squamous cell carcinoma (SCC) and adenocarcinoma, with SCC being more prevalent worldwide except in the USA [12]. The incidence of esophageal SCC varies geographically, with high rates found in certain regions of China, Iran, South Africa, Uruguay, France, and Italy [2]. Risk factors for SCC include low socioeconomic status, tobacco smoking, alcohol consumption, and diet. SCC predominantly affects males and typically occurs in the lower third of the esophagus. Common characteristics of esophageal SCC include stenosis (50% of cases) and visible signs such as infiltration, protrusion, and ulceration [1]. ESCC is a rapidly progressing malignancy with a 5-year overall survival rate ranging from 20-30%. Esophageal pleural fistula is a rare but serious complication of esophageal SCC, characterized by an abnormal communication between the esophagus and the pleural space. It occurs when the tumor infiltrates through the esophageal wall into the adjacent pleura, resulting in the formation of a fistulous tract. This condition poses significant challenges in terms of diagnosis, management, and overall prognosis. The development of an esophageal pleural fistula is associated with advanced-stage esophageal SCC and is often a sign of locally advanced disease or recurrent tumors. Tumor invasion into the pleural space can cause symptoms such as cough, chest pain, dyspnea, recurrent pneumonia, or even hydropneumothorax [13,14]. The two most common types of fistulas are esophagopleural and esophagomediastinal [15,16]. Fistulas may develop when the tumor grows rapidly or when infection hinders normal tissue healing [17]. Risk factors for fistula formation include SCC histology, smoking history, proximal esophageal location, younger age, and a history of esophagectomy or chemotherapy [18]. Malignant esophageal fistulas have an approximate incidence rate of 13%. Diagnosis of an esophageal pleural fistula typically involves a combination of clinical evaluation, imaging studies, and endoscopic procedures. Radiographic modalities like barium swallow, computed tomography (CT) scan, or magnetic resonance imaging (MRI) may be utilized to visualize the fistulous tract, assess tumor extent, and evaluate the involvement of surrounding structures. Endoscopic techniques, including endoscopy and endoscopic ultrasound (EUS), are helpful for the direct visualization and characterization of the tumor, as well as identification of the fistulous connection. A barium swallow is a diagnostic test that involves swallowing a contrast medium (barium sulfate) to visualize the gastrointestinal tract on X-ray images. In the case of an esophageal pleural fistula due to esophageal squamous cell carcinoma (SCC), the barium swallow findings would typically indicate the presence of abnormalities in the esophagus. The specific radiographic findings observed during a barium swallow may vary depending on the severity and location of the fistula, as well as the characteristics of the underlying SCC including contrast leakage from the esophagus into the pleural cavity through the fistula, leading to the visualization of contrast material within the pleural space, abnormal communication between the esophagus and the pleural space, indicating the presence of the fistula, and irregularities or strictures in the esophageal lumen. The barium swallow study of the presented case reveals findings consistent with the presence of the fistula. The imaging shows shoulder and mucosal irregularity in the third segment of the esophagus. Additionally, a small collection of contrast material is observed in the inferior part of the right hemithorax, strongly suggesting the presence of the fistula. These findings on the barium swallow study provides important diagnostic information regarding the extent and location of the esophageal pleural fistula in the context of ESCC. EUS is also a diagnostic procedure that obtains detailed images of the esophagus and surrounding structures. In the case of an esophageal pleural fistula due to SCC, EUS can provide valuable information about the extent and characteristics of the disease. Possible findings are details regarding the size, location, and depth of invasion. Fistula visualization: EUS can help identify the presence and location of the fistulous tract connecting the esophagus and the pleural cavity. The fistula may appear as an abnormal communication between these two structures. EUS can evaluate nearby lymph nodes for any signs of metastasis. Enlarged or abnormal lymph nodes may indicate spread of the SCC. EUS findings, along with clinical and radiological data, can contribute to the staging of the esophageal SCC and help determine the appropriate treatment approach. The histopathology findings of an esophageal pleural fistula due to ESCC can provide important information about the nature and characteristics of the disease. In the context of esophageal SCC with a pleural fistula, the histopathology report may describe the presence of malignant squamous cells within the esophageal tissue. These cells may display varying degrees of differentiation. Evidence of tumor invasion into the surrounding tissues including the esophageal wall, adjacent structures, and potentially the pleural space, identification of a communication tract between the esophagus and the pleural cavity, areas of necrosis within the tumor indicating rapid growth and insufficient blood supply, invasion of tumor cells into blood vessels or lymphatic channels suggesting a higher risk of metastasis, and information about the grade and stage of the SCC determining the aggressiveness of the tumor and the extent of spread are achieved from histopathologic study of the tumor. Management of esophageal pleural fistula in the setting of SCC is challenging and depends on various factors, including the patient’s overall health status, tumor stage, and extent of the fistula. Treatment options may include a combination of palliative measures and interventions aimed at controlling symptoms, such as placement of pleural catheters to drain pleural effusions and provide symptomatic relief. However, curative treatment is often not feasible due to the advanced nature of the disease. In general, the prognosis for patients with esophageal pleural fistula in SCC is poor, primarily due to the advanced stage of the tumor at the time of diagnosis and the associated complications. The presence of an esophageal pleural fistula indicates aggressive disease with a high likelihood of distant metastasis and limited treatment options. It’s essential for patients with esophageal SCC to receive multidisciplinary care involving oncologists, gastroenterologists, thoracic surgeons, and interventional radiologists to determine the most appropriate management strategy based on individual circumstances. Close monitoring, symptom management, and supportive care play crucial roles in improving the quality of life for these patients [6].
In conclusion, EPF represents a significant challenge in the management of patients with ESCC. Early recognition and accurate diagnosis of EPF are essential to facilitate timely intervention. Further research is warranted to explore novel treatment modalities and improve outcomes in this complex clinical scenario.
Acknowledgments
Statement of Transparency and Principals:
· Author declares no conflict of interest
· Study was approved by Research Ethic Committee of author affiliated Institute.
· Study’s data is available upon a reasonable request.
· All authors have contributed to implementation of this research.
References
- Surgical treatment of esophageal carcinoma complicated by fistulas Davydov M, Stilidi I, Bokhyan V, Arzykulov G. European Journal of Cardio-Thoracic Surgery.2001;20(2). CrossRef
- Risk Factors for the Development of Esophagorespiratory Fistula in Esophageal Cancer Paul G, Bohle W, Zoller W. Journal of gastrointestinal and liver diseases: JGLD.2019;28(3). CrossRef
- Survival and prognostic factors of patients with esophageal fistula in advanced esophageal squamous cell carcinoma Guan X, Liu C, Zhou T, Ma Z, Zhang C, Wang B, Yao Y, Fan X, Li Z, Zhang Y. Bioscience Reports.2020;40(1). CrossRef
- Esophageal-Pleural Fistula: the Cause or Effect of Recurrent Pneumonia? Aghdaei HA , Sadeghi A, Nouri G, Salarieh N, Moghadam PK . Case Reports in Clinical Practice.2022. CrossRef
- Esophageal carcinoma Rustgi AK , El-Serag HB . The New England Journal of Medicine.2014;371(26). CrossRef
- Esophagogastropleural fistula secondary to esophageal squamous cell carcinoma: a case report Hsu WH , Wang YT , Huang HC , et al . Medicine (Baltimore).2017;96(28):e7603.
- Esophagopleural Fistula. StatPearls Publishing Banks A, Sherwood K, Frost F. 2021.
- Highlights of the ESMO Consensus Guidelines for the management of patients with metastatic colorectal cancer Lutz MP , Zalcberg JR , Ducreux M, et al . Ann Oncol.2018;29(12):iv132-iv142.
- Management of esophagopleural fistula after esophagectomy for esophageal cancer Lei Y, Jiang B, Ge W, Zhang X. Medicine (Baltimore).2018;97(34):e12107.
- Successful treatment of esophagopleural fistula by covered self-expanding metal stents using intraesophageal stent-fixation technique. Taniyama Y, Nakamura T, Mitamura A, et al . Clin J Gastroenterol.2013;6(3):243-246.
- Clinical Models Of Chemoprevention For The Esophagus Beer DG , Stoner GD . Hematology/Oncology Clinics of North America.1998;12(5). CrossRef
- Randomized study of low-dose versus standard-dose chemoradiotherapy for unresectable esophageal squamous cell carcinoma (JCOG0303) Shinoda M, Ando N, Kato K, Ishikura S, Kato H, Tsubosa Y, Minashi K, et al . Cancer Science.2015;106(4). CrossRef
- Esophageal stenosis and the Glasgow Prognostic Score as independent factors of poor prognosis for patients with locally advanced unresectable esophageal cancer treated with chemoradiotherapy (exploratory analysis of JCOG0303) Okuno T, Wakabayashi M, Kato K, Shinoda M, Katayama H, Igaki H, Tsubosa Y, et al . International Journal of Clinical Oncology.2017;22(6). CrossRef
- Esophagectomy combined with aortic segment replacement for esophageal cancer invading the aorta Cong Z, Diao Q, Yi J, Xiong L, Wu H, Qin T, Jing H, Li D, Shen Y. The Annals of Thoracic Surgery.2014;97(2). CrossRef
- Clinicopathological characteristics and prognosis of patients with esophageal carcinoma invading adjacent structures found during esophagectomy Kosugi S, Ichikawa H, Kanda T, Yajima K, Ishikawa T, Hatakeyama K. Journal of Surgical Oncology.2012;105(8). CrossRef
- Brachytherapy for the palliation of dysphagia owing to esophageal cancer: A systematic review and meta-analysis of prospective studies Fuccio L, Mandolesi D, Farioli A, Hassan C, Frazzoni L, Guido A, Bortoli N, et al . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2017;122(3). CrossRef
- External beam radiotherapy combined with intraluminal brachytherapy in esophageal carcinoma Muijs CT , Beukema JC , Mul VE , Plukker JT , Sijtsema NM , Langendijk JA . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2012;102(2). CrossRef
- Clinical-morphological profiles of esophageal carcinoma's main types Cameniţă D, Demetrian AD , Pleşea RM , Tănasie-Vasile MI , Strâmbu VDE , Grigorean VT , Ioniţă E, Pleşea IE , Marincaş AM . Romanian Journal of Morphology and Embryology = Revue Roumaine De Morphologie Et Embryologie.2020;61(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details