Significance and Impact of Changing WHO Classification Systems on Glial Tumors with Special Reference to IDH1 Mutation in Resource-Limited Setups
Download
Abstract
Objectives: To reclassify glial neoplasms according to the 2021 WHO CNS tumor classification and to study the expression pattern of Isocitrate dehydrogenase 1 (IDH1) mutation by immunohistochemistry(IHC) and to categorize these tumors on the basis of IDH1 mutation.
Methods: This retrospective study included patients who were diagnosed as glial tumors on histology (n=60). A detailed clinical history and radiological findings were obtained. Patients above 18 years were taken as adults while age upto 18 years were included in pediatric group. IHC for IDH1 mutation was done using Anti-IDH1 (R132H) antibody.
Results: Among all glial tumors, 51.7% (31/60) were assigned IDH1 mutant status and the remaining i.e. 48.3% (29/60) were assigned IDH1 wildtype status. While classifying diffuse gliomas according to age, site and morphology, it was observed that the relative proportion of IDH1 mutant and IDH1 wildtype categories in the adult age group was 56.8% (25/44 cases) and 43.2% (19/44 cases) respectively while in the pediatric age group it was 45.5% (5/11 cases) and 54.5% (6/11 cases) respectively. Among the other glial and glioneuronal tumors majority were IDH1 wildtype (80.0%). Most of the WHO grade 1 tumors were IDH1 wildtype (71.4%, 5/7) whereas Grade 2 and 3 tumors were IDH1 mutant (83.3%, 15/18 and 100.0%, 5/5 respectively). On the other hand in Grade 4 tumors there was a higher proportion of neoplasms exhibiting IDH1 wildtype status (70.0%, 21/30).
Conclusion: Identification of IDH status as mutant and wildtype is crucial in glial tumors (especially glioblastoma) because both these groups are clinically, genetically, biologically and prognostically different. IHC provides a standard alternative for molecular studies with high sensitivity and specificity with quick, economical and standard results especially useful in resource-poor countries. Reclassification according to the latest WHO classification appears to confer a overall better prognosis than the previous classifications and this study can also provide a base for future larger research avenues and treatment based on IDH1 mutation.
Introduction
In India, the incidence of central nervous system (CNS) tumors ranges from 5 to 10 per 100,000 population with an upward trend accounting for 2% of malignancies [1]. CNS tumors are associated with very high mortality and morbidity which makes them the most feared form of cancer [2]. Moreover, the difficulty in accessing the CNS tumor tissue, its fragile nature, small specimen size also pose problems in diagnosis [3] apart from the inherent complexities of brain tumors and also lack of uniformity in histological definitions. Glial tumors form a major chunk of this tumor burden.
The integration of phenotypic and genotypic parameters in the latest 2021 World Health Organization (WHO) Classification of Tumors of the Central Nervous System [4], fifth edition, provides increased objectivity which in turn would lead to better correlations not only with the prognosis but also with the treatment response and protocols along with guidelines for investigation of future tailored and targeted therapy. Molecular diagnosis has been given utmost importance so much so as to even supersede the gold standard histology which we have been following since ages [5].
In this era of molecular diagnosis and characterization, emphasis is on molecular subtyping of the CNS tumors but in resource poor setups like ours it is a tough task to achieve. Hence, immunohistochemistry (IHC) can be a very satisfactory surrogate for the same.
Isocitrate dehydrogenase (IDH) 1 mutations occur in a high percentage of diffuse gliomas, with effects on diagnosis, prognosis and treatment, approximately 90% involving exon 4 at codon 132 (R132H) [6] which triggered the basis of integrated genomic-histological diagnosis of brain tumors for the first time in the 2016 WHO classification which has been carried forward by its successor viz. 2021 WHO [7-9].
Aims and Objectives
To reclassify glial neoplasms according to the 2021 WHO classification.
To study the expression pattern of IDH1 mutation in glial neoplasm by IHC and to categorize glial tumors on the basis of IDH1 mutation.
Materials and Methods
Sample Collection
This retrospective study was carried out in the Department of Pathology of a tertiary care centre. CNS space occupying lesion biopsy samples which were diagnosed as glial tumors from July 2016- July 2018 on histology were included. As IDH1 has no role in the characterization of ependymoma, so these cases were excluded. Patients’ age upto 18 years were taken into pediatric group and patients above 18 years were considered as adult group. Total 60 cases were included in the study. A detailed clinical history along with radiological findings including CT (computed tomography) scan and / or MRI (Magnetic Resonance Imaging) were obtained. Biopsy was submitted twice in 2 cases and thrice in one case.
Histopathology samples were collected in 10% buffered formalin and kept for fixation for 12-24 hours. After fixation, representative sections were taken and processed.
Microscopy
For light microscopy, 4-5 micron thick sections were cut and stained with Hematoxylin and Eosin (H&E). The original diagnosis was kept as the provisional diagnosis and the cases were reclassified according to the WHO 2021 classification with the help of IHC as and when required.
Immunohistochemistry - Isocitrate dehydrogenase 1 (IDH1)
First, 2-3 micron sections were taken on Poly-L Lysine coated slides and were incubated for 1 hour at 60-62° C. Slides were then put in xylene for 15-20 minutes followed by hydration with 100%, 90% and 70% alcohol each for 1-2 minutes. The slides were put in running water for 5-10 minutes. Antigen retrieval was done in 2 cycles using citrate buffer at pH = 6.0, first at 95 ° C for 10 minutes and second at 97 ° C for 10 minutes. Slides were left for cooling at room temperature. Washing was done with TRIS (Trisaminomethane) buffer (pH = 7.6); 3 times each for 5 minutes. Peroxidase block was done for 10 minutes. Washing was again done with TRIS buffer. Protein block was done for 10 minutes. Primary antibody
i.e. Anti-IDH1 (R132H) Antibody, Mouse Monoclonal Clone HMab-1, Purified from Hybridoma Cell Culture, Lot#017M4857V, (Sigma-Aldrich) was put on the slide (overnight at 4 ° C) at a dilution of 1: 20. Washing was done with TRIS buffer. Super enhancer was then put and kept for 30 minutes. Washing was done with TRIS buffer. Label (secondary antibody) was kept for 30 minutes followed by washing with TRIS buffer. Diamino-benzidine (DAB) was put and kept for 1-10 minutes until brown color appeared. Slides were then put in distilled water to stop the reaction. Counter staining was done with Harris hematoxylin for 10-15 seconds. Differentiation was done with 1% acid-alcohol. Slides were then blotted, dried and mounted with DPX (Dibutylphthalate Polystyrene Xylene) for light microscopy.
Interpretation of IHC
The antibody for IDH1 stained the cytoplasm and also weakly the nucleus of the tumor cells.[6,10] Both cytoplasmic and nuclear positivity was taken as positive.
[11] A three-tiered semi-quantitative system used in the previous study by Agarwal et al, 2013 was followed [6]:
Negative - if no tumor cell was immunopositive; interpreted as IDH1 wildtype
Focal positivity (partly positive) - if admixture of immunopositive and immunonegative tumor cells were present or there were adjacent areas of immunopositive and immunonegative tumor cells; but this was interpreted as IDH1 wildtype
Diffuse positivity (complete positivity) - if all the tumor cells were immunopositive; interpreted as IDH1 mutant.
Statistical Analysis
Statistical analysis was performed using Statistical Package for Social Science (SPSS, Version 27.0 for windows). Data in the present study was analysed in a descriptive way. The quantitative data was expressed as mean ± SD and qualitative data was expressed in terms of number/ frequency and percentage. p value was calculated using Fisher’s Exact test. Values at ≤ 0.05 were taken as critical level of significance.
Results
Clinical Profile
Out of total 60 cases, 46 were adults and 14 were of the pediatric age group.
For adults, the age ranged between 20-66 years with a mean age of 39.5±03 years and M:F ratio was 1.9:1.
The duration of illness ranged from 1 day to 120 months with mean duration of illness being 19 months.
The age range for pediatric group was 5-18 years with a mean age of 11.7±0.9 years and a M:F ratio of 6:1. The duration of illness ranged from 6 days to 72 months with mean being 11 months.
Headache and vomiting were the most common symptoms in both the groups. Other symptoms included seizures, paresis or paralysis, diplopia, dizziness and fall, loss of consciousness, tingling, numbness, loss of sensation, imbalance, nausea, loss of memory or recognition, slurring of speech etc.
Imaging Studies
On the basis of radiological findings it was found that all the lesions were intracranial and intra-axial in location with majority (83.3%) of them being in the supratentorial region.
Histology and IHC
Histology
While making a histological diagnosis, age of the patient and location of the tumor were taken into account. IDH1 status of the tumor was also given due consideration. Diffuse gliomas were classified into adult-type and pediatric-type. Circumscribed gliomas have been separately classified.
Adult-type diffuse gliomas (n=44):
In nine cases, sections showed a moderately cellular diffuse tumor composed of neoplastic astrocytes having minimal nuclear atypia on a fibrillary background. No atypical mitosis, significant nuclear atypia or microvascular proliferation or necrosis was identified. These cases were categorized as Astrocytoma CNS WHO grade 2 (Figure 1a). Majority of these cases were IDH1 mutant (8/9) (Figure 1d).
Figure 1. a. Astrocytoma WHO Grade 2 Showing Diffuse Infiltrative Neoplastic Astrocytes Having Minimal Nuclear Atypia on a Fibrillary Background (H and E, 20x) b. Astrocytoma WHO Grade 3 displaying increased cellularity with marked atypia (H and E, 20x) c. Astrocytoma WHO Grade 4 showing tumor cells palisading around geographic necrosis (H and E, 20x) d. Astrocytoma WHO Grade 2 tumor cells showing diffuse cytoplasmic and nuclear positivity (IHC IDH1, 20x) e. Astrocytoma WHO Grade 3 tumor cells showing diffuse cytoplasmic and nuclear positivity (IHC IDH1, 20x) f. Astrocytoma WHO Grade 4 tumor cells showing diffuse cytoplasmic and nuclear positivity (IHC IDH1, 20x).
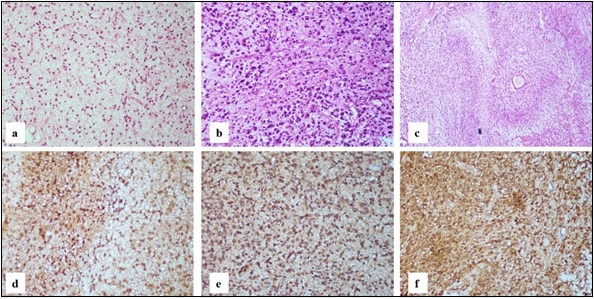
In four cases, biopsy showed moderately cellular diffuse tumor composed of neoplastic astrocytes displaying nuclear pleomorphism, high nuclear cytoplasmic ratio and hyperchromasia. Atypical mitotic activity was noted. No microvascular proliferation or necrosis was identified. These cases were categorized as Astrocytoma CNS WHO grade 3 (Figure 1b). All these were IDH1 mutant (Figure 1e).
Oligodendroglioma CNS WHO grade 2 cases displayed the tumor cells arranged in lobules separated by thin-walled, branching capillaries. Tumor cells had round, uniform nuclei and perinuclear halo with minimal nuclear atypia and mitotic activity. Microcalcification and microcystic changes were present frequently while microvascular proliferation or necrosis was absent.
Most of these cases were IDH1 mutant (4/6).
Oligodendroglioma CNS WHO grade 3 showed a highly cellular tumor composed of atypical cells with round hyperchromatic nuclei, perinuclear halo and prominent nuclear atypia. The characteristic vascular patterns of branching capillaries were present focally. Frequent mitoses were also noted. This case was IDH1 mutant.
Twenty cases were categorized as Glioblastoma grade 4. These tumors were diffusely infiltrating, highly cellular glial tumor composed of tumor cells showing marked nuclear atypia, high nuclear cytoplasmic ratio and marked nuclear pleomorphism with brisk mitotic activity on a fibrillary background. Prominent microvascular proliferation and extensive palisading necrosis were also noted. Out of these cases, eight were IDH1 mutant, so these were reclassified as Astrocytoma, CNS WHO grade 4 (Figure 1c,f) according to the recent WHO classification. The remaining 12 cases which were IDH1 wildtype retained their provisional diagnosis of Glioblastoma CNS WHO grade 4 (Figure 2a,d).
Figure 2. a. Glioblastoma WHO Grade 4 Showing Highly Pleomorphic Bizzare Cells (H and E, 20x). b. Gliosarcoma WHO Grade 4 showing biphasic tumor with glial and mesenchymal elements (H and E, 20x) c. Gliosarcoma – glial component positive for GFAP (IHC GFAP, 20x) d. Glioblastoma WHO Grade 4 tumor cells are negative (IHC IDH1, 20x) e. Gliosarcoma - Vimentin positive in both the glial and mesenchymal components (IHC Vimentin, 20x) f. Gliosarcoma tumor cells are negative (IHC IDH1, 20x) .
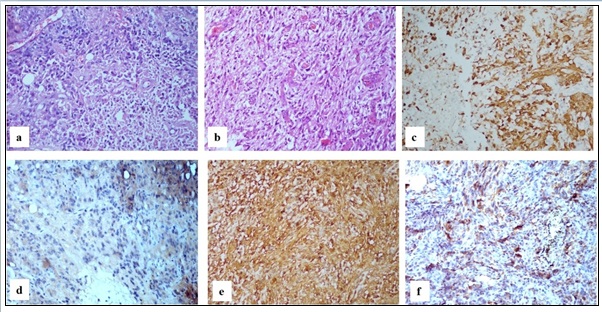
One of the cases showed a highly cellular tumor composed of predominantly plump epithelioid cells with prominent nucleoli and eosinophilic cytoplasm. Brisk mitotic activity was also noted. On IHC the tumor cells were GFAP - Positive; CK - Negative; EMA – Positive. This case was categorized as Epithelioid Glioblastoma CNS WHO grade 4. It was IDH1 wildtype.
Four cases showed morphology of a biphasic tumor with glial and mesenchymal components (Figure 2b). Glial component showed marked nuclear atypia and brisk mitotic activity on a fibrillary background. Prominent microvascular proliferation and palisading necrosis were also noted. Mesenchymal component showed spindle cells in fascicles, mesenchymal component being reticulin positive. On IHC glial component was GFAP positive (Figure 2c), vimentin was positive in both the glial and mesenchymal components (Figure 2e). These four cases were categorized as Gliosarcoma CNS WHO grade 4. In one case, a differential of malignant meningioma was kept which was excluded on the basis of EMA negativity. All were IDH wildtype (Figure 2f).
Pediatric-type diffuse gliomas (n=11):
Five pediatric cases were initially diagnosed as Diffuse Astrocytoma grade 2. This category was further subclassified according to the new classification as follows -
Two cases were diagnosed as Pediatric-type diffuse low-grade glioma, Diffuse Astrocytoma CNS WHO grade 1 showing monomorphic glial tumor cells having bland, round to oval nuclei on a fibrillary background. No mitoses or necrosis were noticed. Among these one was IDH1 mutant and one was IDH1 wildtype.
Three cases showed an infiltrative glial tumor composed of mild to moderate pleomorphic tumor cells with entrapped normal brain tissue. No necrosis, mitoses and/or microvascular proliferation was noted. These were labelled as Pediatric-type diffuse low-grade glioma, Diffuse low-grade glioma CNS WHO grade 2 (Figure 3a). All these were IDH1 mutant (Figure 3d).
Figure 3. Pediatric-type Diffuse Gliomas. a. Diffuse low-grade glioma WHO grade 2 showing an infiltrative glial tumor with mild to moderate pleomorphic tumor cells (H and E, 20x) b. Diffuse Midline Glioma WHO Grade 4 showing anaplastic tumor cells (H and E, 20x) c. Diffuse pediatric-type high-grade glioma WHO grade 4 showing a highly cellular tumor with bizzare cells (H and E, 20x) d. Diffuse low-grade glioma WHO grade 2 tumor cells showing diffuse cytoplasmic and nuclear positivity (IHC IDH1, 20x) e. Diffuse Midline Glioma WHO Grade 4 tumor cells are negative (IHC IDH1, 20x) f. Diffuse pediatric-type high-grade glioma WHO grade 4 tumor cells are negative (IHC IDH1, 20x).
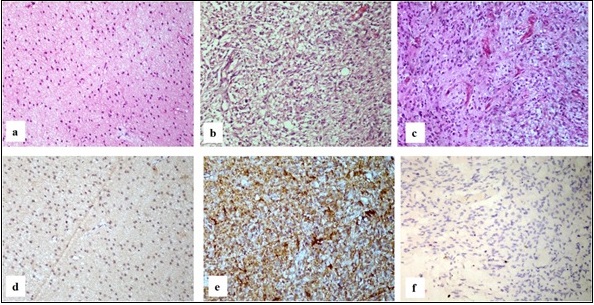
Two cases were diagnosed as Pediatric-type diffuse high-grade glioma, Diffuse midline glioma CNS WHO grade 4. These showed a hypercellular tumor composed of anaplastic and pleomorphic astrocytes with brisk mitoses without microvascular proliferation or necrosis (Figure 3b). One was IDH1mutant and the other was IDH1 wildtype (Figure 3e).
Three cases were diagnosed as Glioblastoma grade 4 which were diffusely infiltrating, highly cellular glial tumor showing marked nuclear atypia, high nuclear cytoplasmic ratio and marked nuclear pleomorphism with brisk mitotic activity on a fibrillary background. Prominent microvascular proliferation and extensive palisading necrosis were also noted. These were reclassified as follows -
Pediatric-type diffuse high-grade glioma, Diffuse hemispheric glioma CNS WHO grade 4 cases showed a highly cellular, infiltrative tumor composed of highly pleomorphic cells having irregular nuclear contour. Microvascular proliferation and necrosis along with brisk mitotic activity were also noted. These were IDH1 wildtype.
Pediatric-type diffuse high-grade glioma, Diffuse pediatric-type high-grade glioma CNS WHO grade 4 showed a highly cellular tumor composed of pleomorphic, bizarre cells with brisk mitoses, microvascular proliferation and palisading necrosis (Figure 3c).This was IDH1 wildtype (Figure 3f).
Other gliomas (n=5):
Sections from cases diagnosed as Pilocytic astrocytoma CNS WHO grade 1 showed a biphasic pattern having bipolar neoplastic cells with elongated, hair-like processes (piloid) projecting from either end and other neoplastic cells with multiple processes on a fibrillary background; variable eosinophilic granular bodies, microcysts and Rosenthal fibres were also seen (Figure 4a). Two were IDH1 wildtype (Figure 4c) while one was IDH1 mutant.
Figure 4. a. Pilocytic Astrocytoma WHO Grade 1 Showing Bipolar Tumor Cells on a Fibrillary Background (H and E, 20x) b. Ganglioglioma WHO Grade 1 showing scattered dysplastic ganglion cells on a background of neoplastic glial cells (H and E, 40x) c. Pilocytic astrocytoma WHO Grade 1 tumor cells are negative (IHC IDH1, 20x) d. Ganglioglioma WHO Grade 1 negative (IHC IDH1, 20x) .
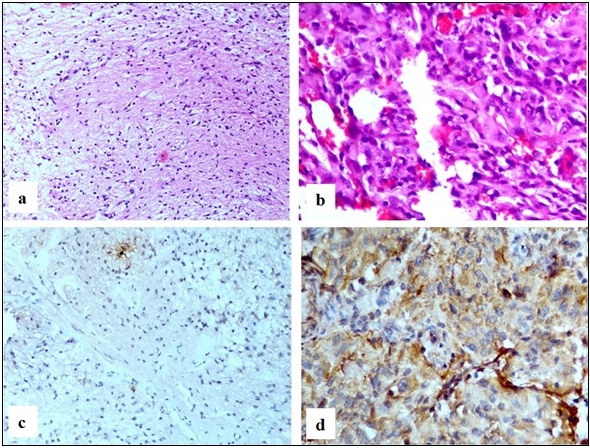
Ganglioglioma CNS WHO grade 1 on microscopic examination showed few scattered dysplastic, large ganglion cells with irregular clustering. Binucleated forms were also seen. Neoplastic glial cells also noted in the background (Figure 4b). One case showed features of Cystic ganglioglioma. Both cases were IDH1 wildtype (Figure 4d).
IHC
The antibody stained the cytoplasm and also weakly the nucleus of the tumor cells. Both cytoplasmic and nuclear positivity was taken as positive. Endothelial cells and residual normal and reactive glial cells did not show immunopositivity. Background staining was absent in most but, wherever present, did not hamper interpretability of results. The observations were made for immunoreactivity of IDH1 as well as of the distribution of IDH1 staining in the section. Accordingly the cases displaying a diffuse immunostaining were assigned an IDH1 mutant status whereas the cases which were either negative or displayed only focal staining for IDH1 were categorized as IDH1 wildtype.
Among all glial tumors, 51.7% (31/60) were assigned IDH1 mutant status and the remaining i.e. 48.3% (29/60) were assigned IDH1 wildtype status.
In concordance with the 2021 CNS WHO classification, while classifying diffuse gliomas according to age, it was observed that the relative proportion of IDH1 mutant and IDH1 wildtype categories in the adult age group was 56.8% (25/44 cases) and 43.2% (19/44 cases) respectively while in the pediatric age group it was 45.5% (5/11 cases) and 54.5% (6/11 cases) respectively.
Table 1 summarizes the result of IDH1 IHC on different adult-type diffuse gliomas while Table 2 summarizes the result of IDH1 IHC on different pediatric-type diffuse gliomas.
| Provisional HPE Diagnosis | Total cases | IDH1 mutant n (%) | IDH1 wildtype n (%) | Final Diagnosis |
| Diffuse Astrocytoma grade 2 | 9 | 8 (88.9) | 1 (9.1) | Astrocytoma CNS WHO grade 2 |
| Anaplastic Astrocytoma grade 3 | 4 | 4 (100) | 0 (0) | Astrocytoma CNS WHO grade 3 |
| Oligodendroglioma grade 2 | 6 | 4 (66.7) | 2 (33.3) | Oligodendroglioma CNS WHO grade 2 |
| Anaplastic Oligodendroglioma grade 3 | 1 | 1 (100) | 0 (0) | Oligodendroglioma CNS WHO grade 3 |
| Glioblastoma grade 4 | 8 | 8 (100) | 0 (0) | Astrocytoma CNS WHO grade 4 |
| Glioblastoma grade 4 | 12 | 0 (0) | 12 (100) | Glioblastoma CNS WHO grade 4 |
| Epithelioid Glioblastoma grade 4 | 1 | 0 (0) | 1 (100) | Epithelioid Glioblastoma CNS WHO grade 4 |
| Gliosarcoma grade 4 | 3 | 0 (0) | 3 (100) | Gliosarcoma CNS WHO grade 4 |
| Total | 44 | 25 (56.8%) | 19 (43.2%) |
| Provisional HPE Diagnosis | Total cases | IDH1 mutant n (%) | IDH1 wildtype n (%) | Final Diagnosis |
| Pediatric-type diffuse low-grade gliomas | ||||
| Diffuse Astrocytoma grade 2 | 2 | 1 (50.0) | 1 (50.0) | Diffuse Astrocytoma CNS WHO grade 1 |
| Diffuse Astrocytoma grade 2 | 3 | 3 (100.0) | 0 (0) | Diffuse low-grade glioma CNS WHO grade 2 |
| Pediatric-type diffuse high-grade gliomas | ||||
| Diffuse midline glioma grade 4 | 2 | 1 (50.0) | 1 (50.0) | Diffuse midline glioma CNS WHO grade 4 |
| Glioblastoma grade 4 | 2 | 0 (0) | 2 (100) | Diffuse hemispheric glioma CNS WHO grade 4 |
| Glioblastoma grade 4 | 1 | 0 (0) | 1 (100) | Diffuse pediatric-type high-grade glioma CNS WHO grade 4 |
| Gliosarcoma grade 4 | 1 | 0 (0) | 1 (100) | Gliosarcoma CNS WHO grade 4 |
| Total | 11 | 5 (45.5%) | 6 (54.5%) |
Among the other circumscribed glial and glioneuronal tumors majority were IDH1 wildtype (80.0%) as shown in Table 3.
| HPE Diagnosis | Total cases | IDH1 mutant n (%) | IDH1 wildtype n (%) |
| Circumscribed astrocytic gliomas | |||
| Pilocytic Astrocytoma CNS WHO grade 1 | 3 | 1 (33.3) | 2 (66.7) |
| Glioneuronal tumors | |||
| Ganglioglioma CNS WHO grade 1 | 2 | 0 (0) | 2 (100.0) |
| Total | 5 | 1 (20.0%) | 4 (80.0%) |
When results were analyzed for various WHO grades (Table 4) for IDH1 status, we observed that most of the WHO grade 1 tumors were IDH1 wildtype (71.4%, 5/7) whereas Grade 2 and 3 tumors were IDH1 mutant (83.3%, 15/18 and 100.0%, 5/5 respectively).
| Grade | Total cases | Adult (n=46) | Pediatric (n=14) | ||
| IDH1 mutant n (%) | IDH1 wildtype n (%) | IDH1 mutant n (%) | IDH1 wildtype n (%) | ||
| CNS WHO grade 1 | 7 | 0 (0) | 2 (100) | 2 (40) | 3 (60) |
| CNS WHO grade 2 | 18 | 12 (80) | 3 (20) | 3 (100) | 0 (0) |
| CNS WHO grade 3 | 5 | 5 (100) | 0 (0) | 0 (0) | 0 (0) |
| CNS WHO grade 4 | 30 | 8 (33.3) | 16 (66.7) | 1 (16.7) | 5 (83.3) |
| Total | 60 | 25 (54.3) | 21 (45.7) | 6 (42.9) | 8 (57.1) |
On the other hand in Grade 4 tumors there was a higher proportion of neoplasms exhibiting IDH1 wildtype status (70.0%, 21/30) and only less than one third expressing IDH1 mutant status (30.0%, 9/30).
Discussion
Many researches are adding evidence to the existing knowledge that IDH1 mutations have a major role in the etiopathogenesis of gliomas; being an early trigger in the tumor genesis pathway [12,13]. Moreover, these mutations also have a major impact on its biological behaviour, clinical progression and prognosis with the conclusion that the mutated tumors show a better prognosis and response to chemotherapy when compared to IDH1/2 wildtype counterparts [14]. The results of meta-analysis of 24 studies done by Chen et al, 2016 [15] indicate that mutations in IDH1 or 2 are associated with improved survival in glioblastomas. It is noteworthy that this is mostly true for adult gliomas [16]. Moreover, status of the IDH mutation might also be related with the expected response to anti-IDH vaccines, medications and treatment [17-19], thus, making IDH the foremost therapeutic biomarker for tailored and individualised treatment as well [9].
The majority (approximately 90%) of glioblastomas occur without a pre-existing, less malignant precursor lesion i.e. they arise de novo and occur in older patients. These are primary glioblastomas and are IDH wildtype [7,20,21]. In contrast, glioblastomas which progress from low-grade diffuse astrocytoma or anaplastic astrocytoma are termed secondary glioblastomas and these occur in younger patients [20]. Also, secondary glioblastomas are associated with a significantly better prognosis than the cases of primary glioblastoma [20]. Data collected from various studies show that most of the secondary glioblastomas are IDH1 mutant accounting for more than 76% whereas only about 5.6% of primary glioblastoma are IDH1 mutant [21,22].
This finding and the ability to observe, follow-up and analyse the overall survival and prognosis on the basis of IDH mutational status has led to a novel observation that many low grade gliomas which are IDH wildtype can be potentially as aggressive as a high grade GBM (glioblastoma multiforme) and have prognosis similar to WHO grade 4 tumors [7]. These low grade IDH wildtype gliomas are referred to as pre-GBM or GBM-like by some authors [21]. Conversely, IDH mutant GBMs have a significantly better prognosis when compared to IDH wildtype GBM and also better than the IDH wildtype lower grade 2 and 3 gliomas [21].
The 2021 WHO CNS tumors classification has taken the morphological and molecular characteristics (mainly IDH status) into consideration while making a diagnosis and simplified classification of adult-type diffuse gliomas only in 3 types – astrocytoma (grade 2,3,4), IDH-mutant; oligodendroglioma, IDH-mutant and 1p/19q-codeleted ; and glioblastoma, IDH-wildtype [4].
So, the importance of testing IDH status of glial tumors cannot be stressed upon more. In this study, we have tried to do so with the best possible resources available and have considered IDH status while making diagnoses. Thus, in the present study, all cases having morphology of glioblastoma along with IDH1 mutant status were diagnosed as Astrocytoma CNS WHO grade 4.
Astrocytoma CNS WHO grade 4, IDH1 mutant cases had longer mean duration of illness (p value 0.042) than the glioblastoma CNS WHO grade 4, IDH1 wildtype cases and this difference was statistically significant, thereby, supporting the diagnosis of secondary GBM according to the previous WHO classification.
The 2021 WHO CNS classification has scrapped off terms like primary GBM, secondary GBM and IDH mutant glioblastoma. There is considerable morphological overlap between astrocytoma CNS WHO grade 4, IDH1 mutant and glioblastoma CNS WHO grade 4, IDH1 wildtype and distinguishing between them requires IDH mutation testing [4]. The major change of reclassification in our study occurred in the glioblastoma category because all the grade 4 IDH1 mutant adult cases were labelled as Astrocytoma grade 4, thus reducing the overall prevalence of glioblastoma cases.
In glioblastoma, IDH-wildtype occurring in younger age, consideration should be given to various types of pediatric-type diffuse high-grade gliomas [5] as done in the present study (Table 2). This has further decreased the total frequency of glioblastoma. Two pediatric diffuse astrocytoma cases on reclassification were down-graded from grade 2 to grade 1 thus, conferring a better prognosis. Overall prognosis according to WHO CNS 2021 will therefore can be assumed to be better than those classified by WHO CNS 2016 [23].
Many studies have shown that pediatric diffuse gliomas differ significantly from their adult counterparts. It has been well established that the biological behaviour and genetic alterations of the two categories are different [24]. Pediatric-type diffuse gliomas are generally indolent despite having histological features of anaplasia and lacking IDH mutation and 1p/19q codeletion which are the genetic hallmarks of adult-type diffuse gliomas. Instead, these have certain characteristic genetic profiles like alteration in the MAPK-pathway [25,26]. The dissection of gliomas at the molecular level has now validated the idea of different age-related pathways in gliomagenesis [27]. Adult diffuse astrocytomas frequently have mutations in IDH1, IDH2, TP53and ATRX which are absent in the pediatric tumors [7, 28]. IDH1 mutations are rare in pediatric gliomas accounting for only 0-17% of cases [16].
The clinical implications of IDH1 mutations in pediatric population is far less understood. It is likely that the IDH1 mutant pediatric tumors will behave differently from the usual indolent nature of most other pediatric low-grade gliomas when observed over long term. It is hypothesized that these tumors are actually adult malignancies which have been diagnosed at a very early stage [16].
The need to classify gliomas into adult-type and pediatric-type has long been considered. The latest 2021 WHO CNS tumors classification has for the first time separated these into distinct categories with the aim of improving care of both children and adults [5]. Although the classification is not dependent on age of the patient. Instead, the classification is based on representative molecular alterations or specific molecular signatures which “primarily” occur in the pediatric and adult age groups respectively, implicating that adult-type gliomas may occur in children and vice versa [4,24,29]. Thus, the 2021 WHO classification of CNS tumors has not specified a certain age cut-off for pediatric and adult diffuse gliomas. As in our study, some of the authors have taken 18 years as the pediatric upper age limit, for example, Ryall et al, 2020 [16] analysed molecular and clinical profiles of 1000 pediatric low-grade gliomas taking age cut-off as less than 19 years [16] while considering site and morphology of the tumors [30].
In pediatric gliomas there are well documented morphologic overlaps not only with their adult counterparts but also within the group [24,29]. Histological classification of pediatric-type diffuse gliomas form a heterogeneous group of glial tumors, the spectrum of which extends from astrocytic and/or oligodendroglial morphology to mixed neuronal-glial morphology. Pediatric-type diffuse low-grade gliomas are graded as 1 and 2 according to the recent WHO classification. They are differentiated from high grade gliomas on the basis of specific histological features or, in case of diffuse gliomas, on the basis of absence of necrosis, mitoses and/or microvascular proliferation [16,31].
When results were analyzed for various WHO grades for IDH1 status we observed that most of the WHO Grade 1 tumors were IDH1 wildtype whereas Grade 2 & 3 tumors were IDH1 mutant. On the other hand in Grade 4 tumors most were IDH1 wildtype. Thus, there was a distinct difference in expression of IDH1 status in various WHO grades. This finding is consistent with the literature which shows that IDH mutation is common in lower grade gliomas (81%) which includes astrocytoma (69%), oligoastrocytoma (87%) and oligodendroglioma (89%) [8,13]. Most of the lower-grade gliomas harbour IDH mutations i.e. in around 65–90% cases [21]. IDH wildtype diffuse astrocytoma and anaplastic astrocytoma are unusual [7]. In contrast, IDH mutation is rare in primary glioblastoma (~8%) [8,13]. In a study done by Deng et al, 2018 [26] to find out association between IDH1/2 mutations and brain glioma grade, they found that IDH mutations were associated with grade 2, 2-3 and 3 gliomas; the mutation frequencies differed significantly between various glioma grades (P<0.001) and these mutations are possibly involved in the progression from grade 2 to grade 3. In their study no IDH mutation was found in grade 1 tumors.
Unfortunately, an entirely molecular approach is presently not always available in routine, daily diagnostic practice, especially in resource-limited settings [24]. Most studies on IDH mutation status are based on DNA sequencing and other molecular studies which are not only labour intensive but also require trained personnel and expensive sophisticated equipment which are not available at every centre. Thus, testing for IDH1 mutation status via IHC is a good and reliable alternative to overcome the above problems. It has the advantage of high sensitivity and specificity, ease of performing the technique and at the same time being cost effective with shorter turn-around-time [11,32,33]. Guidelines for testing IDH status for diagnosis have been established using IHC and DNA sequencing methods [11,34].
Preusser et al, 2011 [34] compared anti-IDH1-R132H IHC and IDH1 gene sequencing and found concordant results in (98.9%) 94/95 cases. Similarly, Loussouarn et al, 2012 [35] compared results of IHC, DNA sequencing and allele-specific PCR for status of IDH1 mutations in gliomas (oligodendrogliomas) and found IHC to be 100% sensitive and 100% specific for R132H mutation in IDH1. Urbanovska et al, 2019 [36] also compared IDH1 mutation status by 2 molecular methods and 2 IHC methods. IHC by one method had a sensitivity of 85.7% and specificity of 100% and IHC by other method showed a sensitivity of 96.4% and specificity of 79.7%. An Indian study done by Agarwal et al, 2013 [6] found concordant results of IHC and DNA sequencing in 88% cases (44/50).
Thus, testing for IDH status via IHC should be performed.
The major limitation of our study was that in our study we did not confirm IDH status by DNA sequencing in IDH negative cases and also cases of glioblastoma were not tested for p53 and EGFR (Epidermal Growth Factor Receptor).
In conclusion, identification of IDH status as mutant and wildtype is of utmost importance in glial tumors (especially glioblastoma) because both these groups are clinically, genetically, biologically and prognostically different, so, their identification and categorization is necessary. IHC provides a standard alternative for molecular studies with high sensitivity and specificity especially in a resource-poor country like ours with quick, economical and standard results.
This is a practical attempt to incorporate the on- going recent advances and developments in the field of neurobiology into our current understanding of glial tumors with a conclusion that reclassification according to the latest WHO classification appears to confer a overall better prognosis than the previous classifications keeping in mind the available sources present in developing countries. This can also provide a base for future larger research avenues and studies including tailored therapy according to the status of IDH mutation for the ultimate benefit of the patients.
Acknowledgments
Statement of Transparency and Principals:
· Author declares no conflict of interest
· Study was approved by Research Ethic Committee of author affiliated Institute.
· Study’s data is available upon a reasonable request.
· All authors have contributed to implementation of this research.
References
- Indian data on central nervous tumors: A summary of published work Dasgupta A, Gupta T, Jalali R. South Asian Journal of Cancer.2016;5(3). CrossRef
- Complications of brain tumors and their treatment Lacy J, Saadati H, Yu JB . Hematology/Oncology Clinics of North America.2012;26(4). CrossRef
- Gliomas: Analysis of disease characteristics, treatment timelines, and survival rates from two tertiary care hospitals of India Mukundan H, Singh S, Lohia N, Taneja S, Sarin A, Bhatnagar S, et al . Clin Cancer Investig J.2020;9:145-154.
- WHO Classification of Tumours Editorial Board World Health Organization Classification of Tumours of the Central Nervous System. 5th ed.Lyon: International Agency for Research on Cancer; 2021..
- The 2021 WHO Classification of Tumors of the Central Nervous System: a summary Louis DN , Perry A, Wesseling P, Brat DJ , Cree IA , Figarella-Branger D, Hawkins C, et al . Neuro-Oncology.2021;23(8). CrossRef
- Comparative study of IDH1 mutations in gliomas by immunohistochemistry and DNA sequencing Agarwal S, Sharma MC , Jha P, Pathak P, Suri V, Sarkar C, Chosdol K, et al . Neuro-Oncology.2013;15(6). CrossRef
- World Health Organization Classification of Tumours of the Central Nervous System. 4th ed., updated ed Louis DN , Ohgaki H , Wiestler OD , Cavenee WK . Lyon: International Agency for Research on Cancer; 2016..
- The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary Louis DN , Perry A, Reifenberger G, Deimling , Figarella-Branger D, Cavenee WK , Ohgaki H, et al . Acta Neuropathologica.2016;131(6). CrossRef
- Isocitrate dehydrogenase (IDH) status prediction in histopathology images of gliomas using deep learning Liu S, Shah Z, Sav A, Russo C, Berkovsky S, Qian Y, Coiera E, Di Ieva A. Scientific Reports.2020;10(1). CrossRef
- Detection of ATRX and IDH1-R132H immunohistochemistry in the progression of 211 paired gliomas Cai J, Zhu P, Zhang C, Li Q, Wang Z, Li G, Wang G, et al . Oncotarget.2016;7(13). CrossRef
- IDH testing in diagnostic neuropathology: review and practical guideline article invited by the Euro-CNS research committee Preusser M, Capper D, Hartmann C. Clinical Neuropathology.2011;30(5). CrossRef
- Comprehensive, Integrative Genomic Analysis of Diffuse Lower-Grade Gliomas Brat DJ , Verhaak RGW , Aldape KD , Yung WKA , Salama SR , Cooper LAD , Rheinbay E, et al . The New England Journal of Medicine.2015;372(26). CrossRef
- Molecular Profiling Reveals Biologically Discrete Subsets and Pathways of Progression in Diffuse Glioma Ceccarelli M, Barthel FP , Malta TM , Sabedot TS , Salama SR , Murray BA , Morozova O, et al . Cell.2016;164(3). CrossRef
- IDH1/2 mutation is a prognostic marker for survival and predicts response to chemotherapy for grade II gliomas concomitantly treated with radiation therapy Okita Y, Narita Y, Miyakita Y, Ohno M, Matsushita Y, Fukushima S, Sumi M, et al . International Journal of Oncology.2012;41(4). CrossRef
- Isocitrate Dehydrogenase (IDH)1/2 Mutations as Prognostic Markers in Patients With Glioblastomas Chen JR , Yao Y, Xu HZ , Qin ZY . Medicine.2016;95(9). CrossRef
- Integrated Molecular and Clinical Analysis of 1,000 Pediatric Low-Grade Gliomas Ryall S, Zapotocky M, Fukuoka K, Nobre L, Guerreiro Stucklin A, Bennett J, Siddaway R, et al . Cancer Cell.2020;37(4). CrossRef
- A vaccine targeting mutant IDH1 induces antitumour immunity Schumacher T, Bunse L, Pusch S, Sahm F, Wiestler B, Quandt J, Menn O, et al . Nature.2014;512(7514). CrossRef
- Pan-mutant IDH1 inhibitor BAY 1436032 for effective treatment of IDH1 mutant astrocytoma in vivo Pusch S, Krausert S, Fischer V, Balss J, Ott M, Schrimpf D, Capper D, et al . Acta Neuropathologica.2017;133(4). CrossRef
- Genotype-targeted local therapy of glioma Shankar GM , Kirtane AR , Miller JJ , Mazdiyasni H, Rogner J, Tai T, Williams EA , et al Proceedings of the National Academy of Sciences of the United States of America.2018;115(36). CrossRef
- The definition of primary and secondary glioblastoma Ohgaki H, Kleihues P. Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2013;19(4). CrossRef
- Glioma Subclassifications and Their Clinical Significance Chen R, Smith-Cohn M, Cohen AL , Colman H. Neurotherapeutics: The Journal of the American Society for Experimental NeuroTherapeutics.2017;14(2). CrossRef
- Genetics and Epigenetics of Glioblastoma: Applications and Overall Incidence of IDH1 Mutation Liu A, Hou C, Chen H, Zong X, Zong P. Frontiers in Oncology.2016;6. CrossRef
- The WHO 2021 Classification of Central Nervous System tumours: a practical update on what neurosurgeons need to know-a minireview Torp SH , Solheim O, Skjulsvik AJ . Acta Neurochirurgica.2022;164(9). CrossRef
- The molecular framework of pediatric-type diffuse gliomas: shifting toward the revision of the WHO classification of tumors of the central nervous system Komori T. Brain Tumor Pathology.2021;38(1). CrossRef
- Pediatric Gliomas: Current Concepts on Diagnosis, Biology, and Clinical Management Sturm D, Pfister SM , Jones DTW . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2017;35(21). CrossRef
- Association between IDH1/2 mutations and brain glioma grade Deng L, Xiong P, Luo Y, Bu X, Qian S, Zhong W, Lv S. Oncology Letters.2018;16(4). CrossRef
- Molecular characteristics of pediatric high-grade gliomas Chamdine O, Gajjar A. CNS oncology.2014;3(6). CrossRef
- Paediatric and adult glioblastoma: multiform (epi)genomic culprits emerge Sturm D, Bender S, Jones DTW , Lichter P, Grill J, Becher O, Hawkins C, et al . Nature Reviews. Cancer.2014;14(2). CrossRef
- The 2021 WHO classification of tumors, 5th edition, central nervous system tumors: the 10 basic principles Komori T. Brain Tumor Pathology.2022;39(2). CrossRef
- Approach to integrating molecular markers for assessment of pediatric gliomas Mahajan S, Sharma MC , Sarkar C, Suri V. International Journal of Neurooncology.2021;4(Suppl 1). CrossRef
- Histologic classification of gliomas Perry A, Wesseling P. Handbook of Clinical Neurology.2016;134. CrossRef
- Characterization of R132H mutation-specific IDH1 antibody binding in brain tumors Capper D, Weissert S, Balss J, Habel A, Meyer J, Jäger D, Ackermann U, et al . Brain Pathology (Zurich, Switzerland).2010;20(1). CrossRef
- Application of mutant IDH1 antibody to differentiate diffuse glioma from nonneoplastic central nervous system lesions and therapy-induced changes Capper D, Sahm F, Hartmann C, Meyermann R, Deimling A, Schittenhelm J. The American Journal of Surgical Pathology.2010;34(8). CrossRef
- Value and limitations of immunohistochemistry and gene sequencing for detection of the IDH1-R132H mutation in diffuse glioma biopsy specimens Preusser M, Wöhrer A, Stary S, Höftberger R, Streubel B, Hainfellner JA . Journal of Neuropathology and Experimental Neurology.2011;70(8). CrossRef
- Comparison of immunohistochemistry, DNA sequencing and allele-specific PCR for the detection of IDH1 mutations in gliomas Loussouarn D, Le Loupp AG , Frenel JS , Leclair François, Von Deimling A, Aumont M, Martin S, Campone M, Denis MG . International Journal of Oncology.2012;40(6). CrossRef
- IDH Mutation Analysis in Glioma Patients by CADMA Compared with SNaPshot Assay and two Immunohistochemical Methods Urbanovska I, Megova MH , Dwight Z, Kalita O, Uvirova M, Simova J, Tuckova L, et al . Pathology oncology research: POR.2019;25(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details