Differences in Nasopharyngeal and Lung Histopathology in Wistar Rats (Rattus norvegicus) Given Inhaled Formaldehyde Exposure with Doses of 20, 30, and 40 ppm
Download
Abstract
Background: Formaldehyde is known as a chemical substance that may induce structural cell changes in several organs, especially the respiratory system. Different concentrations of exposure may result in different degrees of severity in histopathology.
Materials and Methods: Twenty male Wistar rats were divided into 4 groups. Inhalation of 10% formaldehyde exposure for 6 hours per day over 16 weeks was conducted. Nasopharyngeal and lung tissues were collected and stained with hematoxylin-eosin (H&E) to observe histopathological structural changes and assess the degree of severity. Bivariate analysis was performed to calculate correlation coefficients.
Results: This experimental study found that severe dysplasia is correlated with prolonged exposure. A strong correlation was found between formaldehyde concentrations and dysplasia (r=0.682), and a moderate correlation between duration of exposure and dysplasia (r=0.488).
Conclusion: The severity of dysplasia in rats aligns with both the duration and concentrations of formaldehyde exposure. Prolonged exposure increases the severity of dysplasia, as does exposure to higher concentrations of formaldehyde.
Introduction
Malignancy, a global health issue, is prevalent not only in Indonesia but also worldwide. The process of malignancy begins with structural alterations in normal cells, leading to mucosal tissue abnormalities such as hyperplasia, dysplasia, and carcinoma in situ, ultimately progressing to invasive carcinoma. In 2019, Indonesia recorded approximately 270,000 cases of cancer, with nasopharyngeal cancer comprising 5.2% of all cancer cases and a mortality rate of 5.4% among the total cancer population. Conversely, lung cancer ranked as the third most commonly diagnosed cancer (8.8%), following breast cancer and cervical cancer, with a majority of cases occurring in males [1].
Chemical substances, such as formaldehyde, can induce changes in epithelial cells. Formaldehyde, known for its irritant and carcinogenic properties, can be found in various products like disinfectants, floor cleaners, preservatives, and industrial items like wood or carpet coaters [2,3]. While formaldehyde serves as a mediator for purine and amino acid biosynthesis under normal conditions, the human body usually maintains around 100 μM of endogenous formaldehyde. However, it can also be genotoxic and cytotoxic, leading to DNA breakage and chromosomal alterations [4,5]. Prolonged exposure to formaldehyde can result in histopathological changes in some organs. To explore these effects, an experimental study has been undertaken to assess the differences in histopathology and severity between nasopharyngeal and lung tissues after exposure to formaldehyde at various induction concentrations and time periods.
Materials and Methods
This experimental study employed a post-test only experimental design, involving twenty-four male Wistar rats aged 2-3 months and weighing 150-200 grams. The rats were divided into four groups, each consisting of six rats, and were exposed to formaldehyde for 16 weeks, enduring 6 hours of exposure daily. The first group served as the control, while the second, third, and fourth groups were exposed to formaldehyde at concentrations of 20 ppm, 30 ppm, and 40 ppm, respectively. Formaldehyde inhalation was induced using a 10% solution administered with a micropipette and placed in a perforated pot filled with cottons.
To obtain samples from the rats, anesthesia was used in a glass pot containing chloroform-soaked cotton. After a 60-minute anesthesia period, nasopharyngeal and lung tissues were extracted and preserved in cold NaCl solution. Subsequently, fixation was carried out using 4% PBS for 24 hours, followed by soaking in 70% alcohol. Histopathology examinations were conducted using hematoxylin-eosin (H&E) staining. The severity of dysplasia is described in Table 1.
| Grade | Level Involved | Cellular Changes | Architectural Changes |
| Mild (I) | Lower third | -Cell and nucleus pleiomorphism -Hyperchromatic nucleus | -Basal cell hyperplasia |
| Moderate (II) | Up to middle third | -Cell and nucleus polymorphism -Anisonucleosis and anisocytosis -Hyperchromatic nucleus -Elevated mitotic abnormality | -Loss of polarity -Abnormal maturation from basal cell to squamous cell -Elevated cellular density -Basal cell hyperplasia |
| Severe (III) | Up to upper third | -Cell and nucleus polymorphism -Anisonucleosis and anisocytosis -Hyperchromatic nucleus -Elevated mitotic abnormality -Increase cell and nucleus size -Increase size and number of nucleoli | -Nodules -Akantosis -Maturation abnormalities -Elevated cellular density -Basal cell hyperplasia |
For data analysis, univariate analysis was performed on independent and dependent variables using SPSS software for Windows. Spearman’s rho correlation was utilized to determine the level of significance, set at a p-value of <0.05. The study received ethical clearance from Mataram University under the number 316/UN18. F8/ETIK/2023 (Table 2).
| Results | |
| Concentration and dysplasia | |
| Correlation coefficient | 0.682 |
| Significance | 0.001 |
| Duration and dysplasia | |
| Correlation coefficient | 0.488 |
| Significance | 0.029 |
Results
Based on our experimental study, we found that severe dysplasia occurred in rats with long exposure (16 weeks). Table 3 shows the differences between the severity of dysplasia, time exposure, and formaldehyde concentration. The mean age of initial weight is 177.90+2.24 while the mean age before being terminated is 177.15+2.03.
| Group | Weight | Exposure period | Results | |
| Initial Weight | Before Terminated | |||
| Control Group | 165 | 166 | Week 4 | Normal |
| 20 ppm formaldehyde | 175 | 177 | Week 4 | Mild dysplasia |
| 30 ppm formaldehyde | 186 | 187 | Week 4 | Mild dysplasia |
| 40 ppm formaldehyde | 198 | 199 | Week 4 | Mild dysplasia |
| Control Group | 162 | 165 | Week 8 | Normal |
| 20 ppm formaldehyde | 165 | 164 | Week 8 | Mild dysplasia |
| 30 ppm formaldehyde | 163 | 165 | Week 8 | Moderate dysplasia |
| 40 ppm formaldehyde | 174 | 173 | Week 8 | Moderate dysplasia |
| Control Group | 168 | 172 | Week 12 | Normal |
| 20 ppm formaldehyde | 170 | 168 | Week 12 | Moderate dysplasia |
| 30 ppm formaldehyde | 180 | 179 | Week 12 | Severe dysplasia |
| 40 ppm formaldehyde | 193 | 191 | Week 12 | Severe dysplasia |
| Control Group | 183 | 181 | Week 16 | Normal |
| Control Group | 181 | 178 | Week 16 | Normal |
| 20 ppm formaldehyde | 180 | 177 | Week 16 | Severe dysplasia |
| 20 ppm formaldehyde | 181 | 181 | Week 16 | Severe dysplasia |
| 30 ppm formaldehyde | 182 | 179 | Week 16 | Severe dysplasia |
| 30 ppm formaldehyde | 186 | 183 | Week 16 | Severe dysplasia |
| 40 ppm formaldehyde | 182 | 178 | Week 16 | Severe dysplasia |
| 40 ppm formaldehyde | 184 | 180 | Week 16 | Severe dysplasia |
| Mean: 177.90+2.24 | Mean: 177.15+2.03 | |||
| Median: 180.50 | Median: 178.00 |
Figure 1 shows increasing dysplasia severity with higher duration of exposure.
Figure 1. A Chart Illustrating the Distribution of Dysplasia on Different Exposure Period.
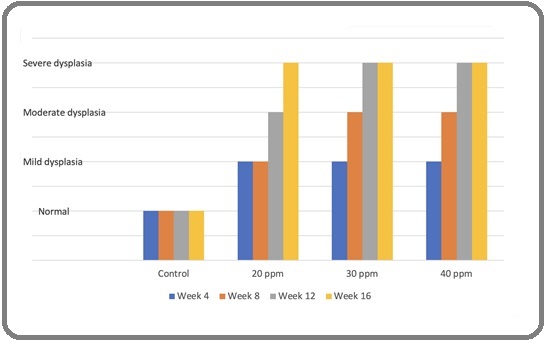
Spearman’s correlation analyses were done, and we found a strong correlation between concentration and dysplasia (r=0.682), and a moderate correlation between duration of exposure and dysplasia (r=0.488). Also, the analyses between concentration and duration of exposure with dysplasia have strong significance (P value < 0.05). Figure 2 shows normal histopathology of nasopharynx and lung.
Figure 2. Normal Histopathology (a) Nasopharynx and (b) Lung.
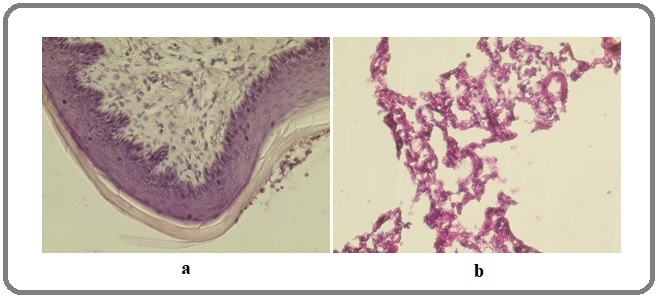
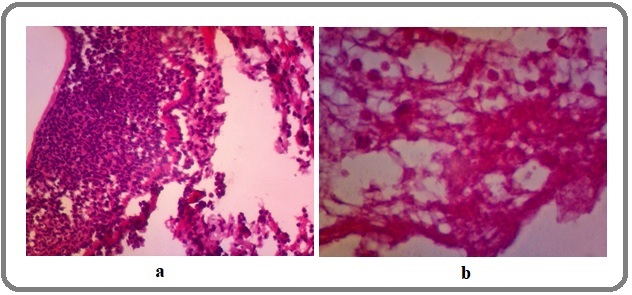
Figure 3 shows severe dysplasia of nasopharynx and lung.
Figure 3. Severe Dysplasia of (a) Lung and (b) Nasopharynx.
Discussion
This research aimed to study the effect of different concentrations and durations of formaldehyde exposure on tissue dysplasia. Formaldehyde exposure has been associated with irritation of the ocular mucosa and skin. Studies using experimental animals, including rats, mice, and monkeys exposed to formaldehyde by inhalation, have shown degeneration and necrosis of respiratory epithelial cells in the nasal cavity [6].
In this research, we found that increasing the concentration of formaldehyde exposure increases the severity of nasopharyngeal and lung tissue dysplasia in rats. We found that in 4 weeks, all concentrations had caused mild dysplasia. Higher concentrations (30 and 40 ppm) caused faster deterioration as they had already caused moderate dysplasia in 8 weeks, compared to the lower concentration. Other studies found different results in different concentrations. Monticello et al. reported that short-term exposure to formaldehyde at concentrations of 6 ppm or higher induced degeneration and necrosis in the nasal cavities of rats. Conversely, exposure to formaldehyde at 2.0 ppm or lower for 5 days per week for 6 weeks did not show any histopathological changes in the nasal cavity. They also found that exposure to 10 and 15 ppm of formaldehyde led to vacuolar degeneration, individual cell necrosis, epithelial exfoliation, and erosion in the respiratory epithelium of the anterior nasal passages after just one day of exposure [7]. Another study found that even at 3 ppm, formaldehyde exposure caused deciliation of cells. Research by Monteiro-Riviere et al. observed cytoplasmic vacuolization in all respiratory epithelia, loss of microvilli in the ciliated respiratory epithelium, and autophagic vacuolization of basal cells in rats exposed to formaldehyde at 6 ppm [8]. Furthermore, Chang et al. exposed mice to 15 ppm of formaldehyde, resulting in early degeneration and sloughing of the respiratory epithelium in the nasal cavity upon immediate sacrifice in rats [9].
In this research, we also found that a longer duration of exposure correlates with increasing dysplasia severity. We found that by 4 weeks of exposure, all rats had developed mild dysplasia. All concentrations showed increasing severity with a longer duration of exposure. By 16 weeks, all rats had developed severe dysplasia. In the literature, various periods (ranging from 1 day to 26 weeks) have been shown to cause cellular changes in the nasopharyngeal tissues. Monticello et al. observed a significant increase in the number of labeled cells in rats exposed to formaldehyde concentrations of 6 ppm or higher for durations ranging from 1 day to 6 weeks. Conversely, no such increase was observed at 2 ppm or lower for any exposure period.7 Zwart et al. found a remarkable ten-fold increase in labeled cells in the respiratory epithelium of the nasal cavity after only 3 days of formaldehyde exposure compared to the control group [10]. Ohtsuka et al. conducted an inhalation study with rats exposed to estimated concentrations of 15–20 ppm for 3 hours per day, 5 days per week, over 2 weeks, leading to the observation of squamous metaplasia in the respiratory epithelium of the nasal cavity [11]. Another research by Rusch et al. studied the exposure of F344 rats, cynomolgus monkeys, and golden hamsters to formaldehyde at 0.19, 0.98, and 2.95 ppm for 22 hours per day, 7 days per week, for 26 weeks. The research reported squamous cell metaplasia of the respiratory epithelium in the nasal cavity of rats and monkeys exposed at 2.95 ppm [12].
In conclusion, this research delved into the impact of different concentrations and durations of formaldehyde exposure on tissue dysplasia, specifically focusing on nasopharyngeal and lung tissues in rats. Our findings demonstrated that increasing formaldehyde concentration led to greater severity of dysplasia. Furthermore, our research highlighted the correlation between longer exposure durations and increasing dysplasia severity.
Acknowledgments
Statement of Transparency and Principals:
· Author declares no conflict of interest
· Study was approved by Research Ethic Committee of author affiliated Institute.
· Study’s data is available upon a reasonable request.
· All authors have contributed to implementation of this research.
References
- World Health Organization. Cancer Indonesia 2020 country profile. 2020 [cited 2023 Aug 3] Available from: https://www.who.int/publications/m/item/cancer-idn-2020..
- Chemicals inhaled from spray cleaning and disinfection products and their respiratory effects. A comprehensive review Clausen PA , Frederiksen M, Sejbæk CS , Sørli JB , Hougaard KS , Frydendall KB , Carøe TK , et al . International Journal of Hygiene and Environmental Health.2020;229. CrossRef
- Formaldehyde carcinogenicity research: 30 years and counting for mode of action, epidemiology, and cancer risk assessment Swenberg JA , Moeller BC , Lu K, Rager JE , Fry RC , Starr TB . Toxicologic Pathology.2013;41(2). CrossRef
- Formaldehyde and De/Methylation in Age-Related Cognitive Impairment Li T, Wei Y, Qu M, Mou L, Miao J, Xi M, Liu Y, He R. Genes.2021;12(6). CrossRef
- Evidence that endogenous formaldehyde produces immunogenic and atherogenic adduct epitopes Nakamura J, Shimomoto T, Collins LB , Holley DW , Zhang Z, Barbee JM , et al . Scientific Reports.2017;7(1). CrossRef
- A comprehensive review of mechanistic insights into formaldehyde-induced nasal cavity carcinogenicity Nishikawa A, Nagano K, Kojima H, Ogawa K. Regulatory toxicology and pharmacology: RTP.2021;123. CrossRef
- Regional increases in rat nasal epithelial cell proliferation following acute and subchronic inhalation of formaldehyde Monticello TM , Miller FJ , Morgan KT . Toxicology and Applied Pharmacology.1991;111(3). CrossRef
- Ultrastructural evaluation of acute nasal toxicity in the rat respiratory epithelium in response to formaldehyde gas Monteiro-Riviere NA , Popp JA . Fundamental and Applied Toxicology: Official Journal of the Society of Toxicology.1986;6(2).
- Nasal cavity deposition, histopathology, and cell proliferation after single or repeated formaldehyde exposures in B6C3F1 mice and F-344 rats Chang JC , Gross EA , Swenberg JA , Barrow CS . Toxicology and Applied Pharmacology.1983;68(2). CrossRef
- Cytotoxic and adaptive effects in rat nasal epithelium after 3-day and 13-week exposure to low concentrations of formaldehyde vapour Zwart A, Woutersen RA , Wilmer JW , Spit BJ , Feron VJ . Toxicology.1988;51(1). CrossRef
- Response of respiratory epithelium of BN and F344 rats to formaldehyde inhalation Ohtsuka R, Shuto Y, Fujie H, Takeda M, Harada T, Itagaki S. Experimental Animals.1997;46(4). CrossRef
- A 26-week inhalation toxicity study with formaldehyde in the monkey, rat, and hamster Rusch GM , Clary JJ , Rinehart WE , Bolte HF . Toxicology and Applied Pharmacology.1983;68(3). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details