Investigating the Properties and Cytotoxicity of Cisplatin-Loaded Nano-Polybutylcyanoacrylate on Breast Cancer Cells
Download
Abstract
Background: This study aimed to develop a novel drug formulation using polybutylcyanoacrylate (PBCA) nanoparticles to deliver cisplatin, a commonly used chemotherapeutic agent for breast cancer treatment.
Materials and Methods: PBCA nanoparticles were synthesized using a mini-emulsion polymerization method, and the resulting NPs were comprehensively characterized for their physical properties, such as size, size distribution, zeta potential, drug loading, and encapsulation efficiency. In addition, the cytotoxicity of the NPs was assessed, along with their ability to release the entrapped drugs over time.
Results: The results showed that the PBCA nanoparticles had a mean size of 457 ± 7.4 nm, a size distribution of 0.253±0.011 and a negative zeta potential of -12.3 ± 1.3 mV. The drug encapsulation efficiency and loading capacity of cisplatin-PBCA were found to be 45.6 ± 2.7% and 3.5 ±0.8%, respectively; The release of the drug from the PBCA was estimated to be approximately 12.2±1.1% after 45 hours. The cytotoxic effects of the nanoparticle formulation were significantly enhanced compared to the free drug. The cytotoxicity of cisplatin-PBCA was evaluated in the T-47D breast cancer cell line, showing promising results as a potential drug formulation for breast cancer therapy.
Conclusions: These findings suggest that cisplatin-PBCA may offer advantages over traditional cisplatin formulations, potentially improving the efficacy and reducing the toxicity of breast cancer treatment.
Introduction
A wide range of diseases shape our modern healthcare landscape. These include complex neurodegenerative disorders like Alzheimer’s, which progressively damages memory and cognitive function, making it difficult to perform daily tasks [1]. There’s also the ongoing global threat of infectious diseases like COVID-19, which has had a profound impact on public health [2, 3]. Furthermore, we must consider the persistent burden of chronic conditions such as cirrhosis, a late-stage liver scarring often caused by long-term liver damage from factors like chronic alcohol abuse or viral infections [4, 5]. Hepatitis C virus, a bloodborne pathogen, infects the liver, leading to inflammation and potentially cirrhosis and liver cancer if not treated [6]. High blood pressure or hypertension is a chronic medical condition characterized by consistently elevated blood pressure against the walls of the arteries [7]. This condition significantly raises the risk of heart disease, stroke, and various other health complications. Vertigo is a disorienting sensation of dizziness or spinning, often accompanied by a feeling of imbalance, and it can result from various underlying medical conditions or disturbances in the inner ear [8]. Cancer is a complex group of diseases characterized by the uncontrolled growth and spread of abnormal cells, and it encompasses a wide variety of types such as breast cancer, lung cancer [9], prostate cancer [10], pancreatic cancer [11], gastric cancer [12], brain cancer [13], and many more. Among these cancers, breast cancer, marked by abnormal cell growth in breast tissue, illustrating the diverse range of health problems that require medical attention and research in our complex healthcare system [14, 15].
Breast cancer is a leading cause of death among women worldwide, and its complexity arises from both genetic and environmental factors. While age, gender, family history, obesity, diet, race, and other factors can increase cancer risk, the exact etiology of breast cancer remains unclear. Treatment options for breast cancer include surgery, radiation therapy, chemotherapy, hormone therapy, and others. Although chemotherapy is an effective treatment option, its toxic side effects, such as liver and kidney damage, immunosuppression, vomiting, hair loss, and more, can be significant limitations for patients. In addition, damage to normal tissues caused by tumor drugs is a major concern for individuals with breast cancer [16-18]. Cisplatin is a widely used chemotherapy drug effective against various types of cancer, including breast cancer. It works by binding to DNA molecules, which leads to the induction of programmed cell death (apoptosis) or accidental cell death (necrosis) in cancer cells. However, its use can also result in adverse side effects, such as kidney damage (nephrotoxicity) and nerve damage (neurotoxicity) [19]. The utilization of nanotechnology materials for the delivery of chemotherapeutics holds great promise, as it can significantly improve drug penetration into tumors, augment targeting efficiency, and concurrently minimize adverse side effects [20, 21]. Polymeric nanoparticles can be produced using two different polymerization techniques: dispersion polymerization and emulsion polymerization. PBCA NPs have several advantages, such as the capacity to change the distribution of medicines within the body, biodegradability, and simplicity of synthesis and purification. Mini-emulsion polymerization and anionic polymerization are the two most popular ways to make PBCA NPs. With this approach, there is no need to move monomers or other hydrophobic substances from one container to another during polymerization because the droplets serve as both the beginning and the growth of the polymerization sites, making mini-emulsion polymerization a one-stage nano-encapsulation method for encapsulating hydrophobic molecules [22].
Cisplatin is a commonly used chemotherapy drug for treating various types of cancer, including breast cancer. Despite its effectiveness, it has been challenging to develop a suitable nanoparticle formulation of cisplatin due to its hydrophilic nature. Several methods and nanomaterials are available today for treating cancer and other serious diseases. The combination of iron nanoparticles with metal-organic- frameworks and azathioprine has been remarkably effective in drug delivery [23]. In this study, we investigated the use of PBCA-nanoparticles as a carrier system for delivering cisplatin. We employed mini-emulsion polymerization to load cisplatin onto PBCA-nanoparticles and evaluated their efficacy in vitro using the T-47D cell line of breast cancer cells that exhibit resistance to cisplatin. Our findings suggest that the developed NPs could potentially enhance the therapeutic index of cisplatin and overcome resistance in breast cancer treatment.
Materials and Methods
Butyl cyanoacrylate monomer was purchased from Evobond® Tong Shen Enterprise Co., Ltd., Taiwan. Cisplatin, dextran 7000, and polyethylene glycol 400 were obtained from Sigma-Aldrich Co., UK. Hydrochloric acid and sodium hydroxide were supplied by Merck Company. The T-47D cell line was obtained from the cell bank of the Iran Pasteur Institute.
Preparation method for NPs containing drugs
To prepare NPs containing drugs, a combination of butyl cyanoacrylate monomers, HCl, honey, olive oil, dextran, PEG, and cisplatin were used. First, 330 μL of butyl cyanoacrylate monomers were mixed with 300 μL of HCl 0.01N, 130 mg of honey, 35 μL of olive oil, and 55 mg of dextran in a reaction vessel. Subsequently, 90 mg of PEG and 60 mg of cisplatin were added to the mixture. The components were thoroughly mixed under laboratory conditions using a stirrer at 150 rpm. Next, 35 mL of cold distilled water was gradually added to the mixture over two steps while continuously stirring the mixture at 400 rpm for 10 minutes. To create a pre-emulsion, the mixture was then subjected to sonication using a probe sonicator (50 W, Bandel in Sonopuls HD 2070, Bandelin Elec., Germany) with the flask placed in an ice bath. The emulsion was left to settle for 24 hours at 4°C before being transferred back onto the stirrer and gently agitated (150 rpm, 3.5 hours) to allow the polymerization process to complete. Finally, the pH of the mixture was adjusted to neutral using NaOH 0.1N.
Characterization of PBCA
The characterization of PBCA involved determining their mean size, size distribution, and zeta potential using a Zetasizer (Nano ZS3600, Malvern Instruments of UK).
In vitro study of drug release
In vitro, drug release studies were conducted using 80 mg of centrifuged drug-loaded nanoparticles (NPs) redispersed in 6.5 mL of fresh human serum in a capped centrifuge tube. The tube was incubated at 37°C while shaking at 120 cycles per minute using a Kühner shaker incubator (Birsfelden, Switzerland). At predetermined time points, the tube was centrifuged at 21,000 rpm and 4°C for 60 minutes, and the supernatant was removed.
The collected NPs were then redispersed in 6.5 mL of fresh human serum for continued release study. The amount of cisplatin released into the supernatant was determined using ICP-EOS elemental analysis.
Determination of encapsulation efficiency and drug loading
To evaluate the drug loading efficiency and encapsulation of the nanoparticles, we employed two mathematical formulas. We first centrifuged the emulsion of NPs at 21,000 rpm and 4°C for 50 minutes to separate the particles from the solvent. The amount of drug in the supernatant was then determined using Inductively Coupled Plasma Optical Emission Spectrometry (ICP-OES) elemental analysis with a 730-OES instrument from Varian. This allowed us to calculate the drug loading efficiency and encapsulation efficiency of the nanoparticles.
Encapsulation (%)= (Amount of drug in carrier (mg/ml) / (Amount of drug fed initially (mg/ml)) ×100
Loading efficiency (%)= (Amount of drug in nanoparticle (mg/ml) / (Weight of nanoparticle (mg/ml)) ×10
Cytotoxicity assay
The viability of T-47D cells was evaluated using an MTT assay, which involved seeding the cells in a 96-well plate at a density of 1×104 cells per well and culturing them in a controlled atmosphere with 5% CO2 at 37°C in RPMI-1640 culture medium supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin antibiotics. The cells were allowed to attach for 24 hours before treating them with varying concentrations of free cisplatin and cisplatin-PBCA. The viability of the cells was then assessed over a period of 48 hours, and the absorbance was measured at 570 nm using an ELISA reader (BioTek Instruments, VT, USA). The IC50 value was determined using the statistical package Pharm-PCS software (Springer Verlag, USA).
Statistical analysis
The results are presented as means ± SD (n = 3). We used one-way ANOVA in IBM Statistics SPSS software version 19 for statistical analysis, and set statistical significance at p < 0.05.
Results
Characterization of nanoparticles
The size, polydispersity index (PDI), and zeta potential of the PBCA were determined to be 457 ± 7.4 nm, 0.253±0.011 and -12.3 ± 1.3 mV.
In vitro study of drug release
The cumulative release rate of Cisplatin from Cisplatin-PBCA nanoparticles was depicted in Figure 1.
Figure 1. Release Pattern of Cisplatin from Cisplatin-PBCA NPs Synthesized by Miniemulsion Polymerization. The pattern is presented as a percentage of release in different time intervals.
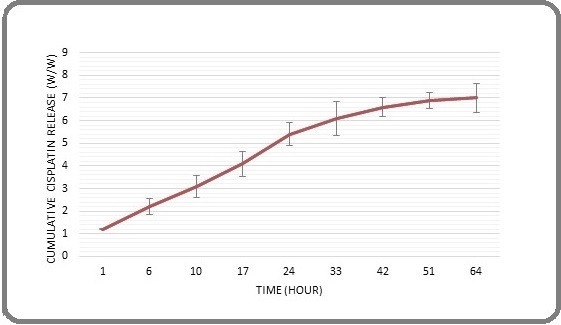
The results showed that the nanoparticles had a high drug retention capacity, with only 7.1% (w/w) of the drug being released after 64 hours. Initially, within the initial 17 hours of the assessment, a rapid liberation of the medication was noticed, which may have been due to the release of adsorbed drug molecules on the surface of the NPs. Subsequently, the release rate slowed down significantly over time, with only 0.35% of the drug being released during the final 24 hours of the evaluation period. It is likely that the presence of PEG contributed to the reduced drug release, as it can form a protective layer around the NPs and hinder drug diffusion.
Determining Drug Loading and Encapsulation Efficiency
To determine the amount of drug loaded into the nanoparticles and the encapsulation efficiency, we used a standard curve for drug formulation. We found that the encapsulation percentage was 40.6 ± 2.7% and the loading efficiency was 3.3 ±0.8%.
In vitro viability
The in vitro viability of cisplatin-PBCA and free cisplatin was evaluated using an MTT assay in T-47D cells. The half-maximal inhibitory concentration (IC50) of cisplatin-PBCA and free cisplatin for T-47D cells is depicted in Table 1.
| Cell line | IC 50 of cisplatin | IC 50 of cisplatin-PBCA | P value |
| T-47D | 84.2±7.1 | 48.5±4.4 | p < 0.05. |
The results showed that both free cisplatin and cisplatin-PBCA displayed dose-dependent cytotoxicity against this cell line, with cisplatin-PBCA demonstrating greater efficacy against tumor cells compared to free cisplatin.
Data are expressed as mean ± SD from three independent experiments.
Discussion
In this study, we successfully synthesized PBCA NPs containing a high level of cisplatin using miniemulsion polymerization, which proved to be a reliable and effective method. We conducted the experiment three times to ensure the results were consistent and reliable. Our findings demonstrated that the production of NPs involving butyl cyanoacrylate monomer was influenced by various factors, including monomer concentration, stabilizer or dextran concentration, pH environment, and rapid stirring. Notably, we utilized a combination of honey and olive oil as a surfactant in the manufacturing process, which contributed to the anti-cancer properties of the NPs [22]. Sreelakshmi et al. discovered that gold and silver nanoparticles (NPs) coated with honey exhibit exceptional antimicrobial properties [24]. Similarly, Wu et al. found that naked carbon NPs derived from commercial food-grade honey showed improved imaging capabilities for sentinel lymph nodes [25]. Additionally, researchers have observed similar positive effects when using olive oil as a coating material for NPs [26]. The PEG used in this study has several advantages that contribute to its effectiveness in cancer treatment. Firstly, it can enhance the stability of drugs and improve their delivery to tumors [27]. Additionally, PEG has high water solubility, which reduces the risk of immune response and antigenicity, making it less likely to trigger an adverse reaction [28]. Furthermore, PEG can prolong the duration of drug release, allowing for more efficient treatment over time. It’s possible that the presence of PEG in the nanoparticle structure contributes to its ability to retain drugs and release them slowly, further enhancing its therapeutic potential.
The zeta potential measurement of -12 mV confirmed the stable dispersion of nanoparticles. The high encapsulation capacity of up to 45% demonstrated the effectiveness of the preparation method. In addition, the drug release study showed that PBCA nanoparticles were capable of retaining cisplatin, but a burst release occurred within 17 hours, suggesting the release of adsorbed drug from the particles. The low and slow release of drug from nanoparticles may be attributed to the presence of PEG in the formulation, as previously reported by ebrahimi far et al [18]. Notably, PEG not only improves the stability of nanoparticles but also enhances their ability to deliver drugs to tumors, ultimately increasing the therapeutic efficacy of the drug. Different studies have shown that polymeric nanoparticles can decrease drug release into the external environment [22].
The MTT assay is a widely used method for evaluating cell viability and toxicity, and thus it was employed in this study to investigate the cytotoxic effects of nano-cisplatin [29]. The results demonstrated that nanoparticle-mediated delivery of cisplatin resulted in enhanced cytotoxicity towards T-47D breast cancer cells, as evidenced by a lower IC50 value compared to free cisplatin. This suggests that the nanoparticle formulation potentiates the anticancer activity of cisplatin. However, further research is necessary to optimize the efficacy of this novel drug delivery system and explore its potential for treating various types of cancer. In this regard, strategies such as incorporating folate ligands, dextran, or monoclonal antibodies into the nanoparticle formulation may be worth investigating.
In summary, the findings of this study indicate that Poly Butyl Cyanoacrylate nanoparticles can effectively enhance the cytotoxicity of cisplatin against breast cancer cell line T-47D, with an IC50 value of 48 μM being observed for the nanodrug, which is significantly lower than the IC50 value of 84 μM for free cisplatin. To the best of our knowledge, this is the first study to evaluate the efficacy of cisplatin-loaded Poly Butyl Cyanoacrylate nanoparticles on the T-47D cell line. These results provide valuable insights into the potential of nanoparticle-based drug delivery systems for improving the therapeutic index of chemotherapeutics like cisplatin.
In conclusion, our findings suggest that PBCA nanoparticles are an effective carrier for delivering cisplatin to breast cancer cells. Our research indicates that cisplatin loaded onto these nanoparticles exhibits greater cytotoxicity compared to free cisplatin against brain tumor cells. Therefore, this formulation could potentially serve as a promising chemotherapy option for treating brain cancer in the future.
Co-Author Contributions
Authors’ contribution Amirsasan Gorgzadeh, Ali Heidari, Parizad Ghanbarikondori, and Zeinab Jabbari Velisdeh performed the experimental tests. Elham Saberian, and Somayeh Eslami designed the nanoparticle. Ali Habiba did the drug release test. Cell culture was carried out by Mahshid Arastonejad and Paniz mirmoghaddam. Tayebeh Ghasemi Goki, and Mehrad Aria performed the statistical analysis. Armin Sedighi set up and performed the work with the laboratory devices. Ahmadreza Allahyartorkaman, and Farimah Moazzam wrote the article.
Data availability
Not applicable as we used information from previously published articles.
Approved by any scientific Body
Not applicable as the manuscript is not a part of any student thesis or study.
Ethical issue and approval
Not applicable as we used information from previously published articles.
Consent for publication
All authors have given consent for publication.
Conflict of interest
The authors declare no potential conflict of interest.
References
- Preparation and characterization of brain-targeted polymeric nanocarriers (Frankincense-PMBN-lactoferrin) and in-vivo evaluation on an Alzheimer's disease-like rat model induced by scopolamine Moazzam F, Hatamian-Zarmi A, Ebrahimi Hosseinzadeh B, Khodagholi F, Rooki M, Rashidi F. Brain Research.2024;1822. CrossRef
- The Effectiveness of Existential Therapy Intervention on Anxiety Caused by Coronavirus and Death Sabzevari P, Abady FHE , Araghian S, Bahramian F, Isanezhad A. Clinical Cancer Investigation Journal.2022;11(1s):1-7.
- COVID-19 Lung CT Scans: A large dataset of lung CT scans for COVID-19 (SARS-CoV-2) detection.2021 Kaggle Aria M, Ghaderzadeh M, Asadi F, Jafari R. . CrossRef
- Performance of machine learning techniques on prediction of esophageal varices grades among patients with cirrhosis Bayani A, Asadi F, Hosseini A, Hatami B, Kavousi K, Aria M, Zali MR . Clinical Chemistry and Laboratory Medicine.2022;60(12). CrossRef
- Identifying predictors of varices grading in patients with cirrhosis using ensemble learning Bayani A, Hosseini A, Asadi F, Hatami B, Kavousi K, Aria M, Zali MR . Clinical Chemistry and Laboratory Medicine.2022;60(12). CrossRef
- The Prevalence of Hepatitis C Virus Infection in Patients With Thalassemia in Zabol City of Iran Yousefi M, Dehesh MM , Ebadi M, Dehghan A. International Journal of Infection.2017;4(1). CrossRef
- Oncohypertension; treatment of high blood pressure in cancer patients Alem L, Esmaeil pour MA , Borja Montes OF , Khayyat A, Kaviani P, Ebadi M. J Nephropathol.2023;12(4):e21513-e. CrossRef
- Diagnostic Accuracy of Neuron Specific Enolase (NSE) and S100B in Distinguishing Central from Peripheral Vertigo Masoumi B, Bagheri R, Heydari F, Golshani K, Ansari B, Khatami M. The Journal of Emergency Medicine.2017;53. CrossRef
- New bidirectional recurrent neural network optimized by improved Ebola search optimization algorithm for lung cancer diagnosis Sabzalian MH , Kharajinezhadian F, Tajally A, Reihanisaransari R, Ali Alkhazaleh H, Bokov D. Biomedical Signal Processing and Control.2023;84. CrossRef
- Fabrication of Antibody Conjugated Super Magnetic Oxide Nanoparticles for Early Detection of Prostate Cancer Nayerpour Dizaj T, Jafari-Gharabaghlou D, Farhoudi Sefidan Jadid M, Jahanban R, Rahimi M, Farajollahi MM , Mohsenzadegan M, Zarghami N. Asian Pacific Journal of Cancer Prevention.2023;24(6). CrossRef
- The circadian clock as a potential biomarker and therapeutic target in pancreatic cancer Pourali G, Ahmadzade AM , Arastonejad M, Pourali R, Kazemi D, Ghasemirad H, Khazaei M, et al . Molecular and Cellular Biochemistry.2023. CrossRef
- Characteristics and Cytotoxic Effects of Nano-Liposomal Paclitaxel on Gastric Cancer Cells Abedi Cham Heidari Z, Ghanbarikondori P, Mortazavi Mamaghani E, Hheidari A, Saberian E, Mozaffari E, Alizadeh M, Allahyartorkaman M. Asian Pacific Journal of Cancer Prevention.2023;24(9). CrossRef
- Toxicity of Carboplatin-Niosomal Nanoparticles in a Brain Cancer Cell Line Abbasi M, Abedi Cham Heidari Z, Poustchi F, Hheidari A, Ghanbarikondori P, Salehi H, et al . Asian Pacific Journal of Cancer Prevention: APJCP.10;24.
- Time-related survival prediction in molecular subtypes of breast cancer using time-to-event deep-learning-based models Zarean Shahraki S, Azizmohammad Looha M, Mohammadi Kazaj P, Aria M, Akbari A, Emami H, Asadi F, Akbari ME . Frontiers in Oncology.2023;13. CrossRef
- Development of PEGylated PLGA Nanoparticles Co-Loaded with Bioactive Compounds: Potential Anticancer Effect on Breast Cancer Cell Lines Mohammadinejad S, Jafari-Gharabaghlou D, Zarghami N. Asian Pacific journal of cancer prevention: APJCP.2022;23(12). CrossRef
- Phycosynthesis of Silver Nanoparticles Using Cladophora Glomerata and Evaluation of Their Ability to Inhibit the Proliferation of MCF-7 and L20B Cell Lines Fayyad RJ , Mohammed Ali AN , Saeed NAAAH , Hamzah IH , Dwaish AS . Asian Pacific journal of cancer prevention: APJCP.2022;23(10). CrossRef
- Design and Development of Nanostructured Co Delivery of Artemisinin and Chrysin for Targeting hTERT Gene Expression in Breast Cancer Cell Line: Possible Clinical Application in Cancer Treatment Khoshravan Azar L, Dadashpour M, Hashemi M, Zarghami N. Asian Pacific Journal of Cancer Prevention : APJCP.2022;23(3). CrossRef
- Preparation, Characterization and Cytotoxicity of Silibinin- Containing Nanoniosomes in T47D Human Breast Carcinoma Cells Amiri B, Ebrahimi-Far M, Saffari Z, Akbarzadeh A, Soleimani E, Chiani M. Asian Pacific journal of cancer prevention: APJCP.2016;17(8).
- Effects of Cisplatin-Loaded Niosomal Nanoparticleson BT-20 Human Breast Carcinoma Cells Kanaani L, javadi I, Ebrahimifar M, shahmabadi HE , Khiyavi AA , Mehrdiba T. Asian Pacific Journal of Cancer Prevention : APJCP.2017;18(2). CrossRef
- The protective effect of Nigella sativa against cisplatin-induced nephrotoxicity in rats Hosseinian S, Khajavi Rad A, Hadjzadeh MAR , Mohamadian Roshan N, Havakhah S, Shafiee S. Avicenna Journal of Phytomedicine.2016;6(1).
- Lutein with various therapeutic activities based on micro and nanoformulations: A systematic mini-review Maghsoudloo M, Bagheri Shahzadeh Aliakbari R. Micro Nano Bio Aspects.2023;2(4). CrossRef
- General Characteristics and Cytotoxic Effects of Nano-Poly (Butyl Cyanoacrylate) Containing Carboplatin on Ovarian Cancer Cells Kanaani L, Ebrahimi Far M, Kazemi SM , Choupani E, Mazloumi Tabrizi M, Ebrahimi Shahmabadi H, Akbarzadeh Khiyavi A. Asian Pacific journal of cancer prevention: APJCP.2017;18(1). CrossRef
- Synthesis of an organic metal nanoporous structure for controlled azathioprine delivery Maghsoudloo M, Abdouss M, Kowsari E. Nexo Revista Científica.2021;34. CrossRef
- Honey derivatized Au and Ag nanoparticles and evaluation of its antimicrobial activity Sreelakshmi C., Datta KKR , Yadav JS , Reddy BVS . Journal of Nanoscience and Nanotechnology.2011;11(8). CrossRef
- A Green Synthesis of Carbon Nanoparticle from Honey for Real-Time Photoacoustic Imaging Wu L, Cai X, Nelson K, Xing W, Xia J, Zhang R, Stacy AJ , et al . Nano research.2013;6(5). CrossRef
- (PDF) Synthesis and Characterization of Olive Oil Mediated Iron Oxide Nanoparticles Palanisamy K, Meenakshi Sundaram N, Devabharathi V, Thangarasu P. Digest Journal of Nanomaterials & Biostructures (DJNB).2013;8(2).
- PEGylated nanoparticles for biological and pharmaceutical applications Otsuka H, Nagasaki Y, Kataoka K. Advanced Drug Delivery Reviews.2003;55(3). CrossRef
- Anti-cancer activity of pegylated liposomal trans-anethole on breast cancer cell lines MCF-7 and T47D Shahbazian S, Akbarzadeh A, Torabi S, Omidi M. Biotechnology Letters.2015;37(7). CrossRef
- In vitro evaluation of the effects of acetone, on the potency of cisplatin: Is it a good candidate for cisplatin carrier preparation? Shahmabadi HE , Akbarzadeh A, Mokhtari MJ , Mortazavi M, Ghasemi S, Mohammadi H, Doun SKB . E3 J Biotechnol Pharm Res.2012;3:137-140.
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2023
Author Details