A Case of Dynamic Molecular Profile in Metastatic Lung Adenocarcinoma and the Significance of Multiple-site Testing
Download
Abstract
Lung adenocarcinomas show intratumoral heterogeneity which has significant impact on the selection of targeted therapy. Multiple molecular sub-clones coexist within an individual tumour which determine recurrence and metastasis formation which is often under-evaluated in a single-site needle biopsy. Our case report demonstrates this intriguing phenomenon and the importance of multiple-site molecular testing to determine the dominant molecular alteration, and initiating the appropriate targeted therapy for better prognosis. Molecular testing from accessible metastatic sites in lung cancer is recommended for a complete assessment of prognosis and therapeutic options in recurrent and metastatic disease.
Introduction
Non-small cell lung cancer has a complex biology with a heterogenous landscape of molecular clones, many of which are targets for personalized therapies [1, 2]. Lung adenocarcinomas have demonstrated striking histological and molecular heterogeneity within the same tumor which has significant impact on the selection of target-specific tyrosine-kinase inhibitors [3-5]. Often populations of several sub-clones coexist within an individual tumour which determine recurrence and metastasis formation, thus characterizing a dismal prognosis [6, 7]. Also, a selection pressure for pre-existing drug-resistant sub-clones to triumph, accounts for development of secondary resistance to targeted therapy [3,8]. The main challenge against revealing intratumoral heterogeneity and determining the most compelling molecular clones that determine the disease outcome, is the limited sampling area of the primary tumour and paucity of the available material for initial molecular testing. A single site, needle biopsy grossly underestimates the spatial-temporal heterogeneity of a tumor. This often results in missing the driver mutation or the most culpable mutation that would signal the advent of an aggressive and perilous disease course [9, 10]. This can have enormous implications for a patient when major therapeutic decisions are made with limited and incomplete pathological information [11, 12]. Here, we describe a case which was a fascinating demonstration of this intriguing biological behavior of non-small cell lung cancer and how timely and appropriate therapeutic intervention caused a dramatic response in the disease course.
Case presentation
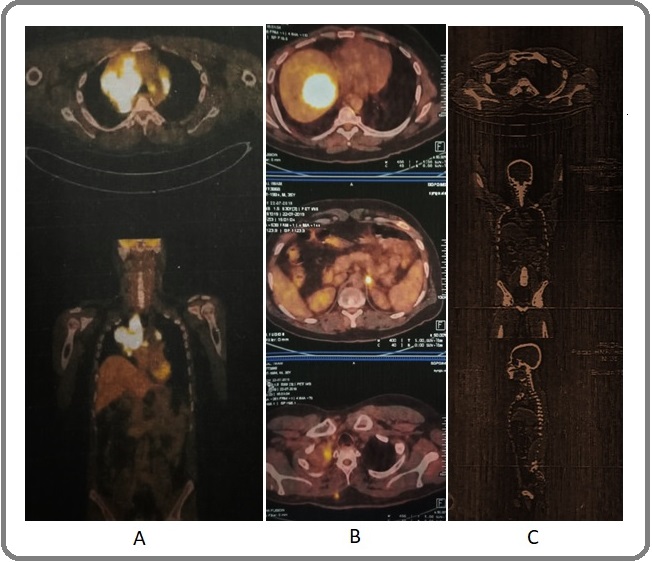
A 30-year-old, never smoking male was diagnosed as a case of non-small cell lung carcinoma of the right lung in November 2015. PET-CT scan showed a hypermetabolic right hilar lesion (SUV 14.07) (Figure 1A).
Figure 1. A. PET-CT (2015), hypermetabolic right hilar lesion, SUV 14.07. B. PET-CT (2019), Left adrenal lesion, soft tissue lesion (disease progression). C. PET-CT (2021), decrease in SUV of right lung lesion (4.1), complete regression of mediastinal nodes, decrease in left adrenal and liver lesions .
Histopathology was poorly-differentiated adenocarcinoma (Figure 2A) and molecular testing for EGFR mutation was wild type.
Figure 2. A. Right lung needle biopsy, poorly- differentiated adenocarcinoma, H and E, 200X. B. Fine-needle aspirate of soft tissue lesion on the back, metastatic adenocarcinoma, MGG stain, 200X.
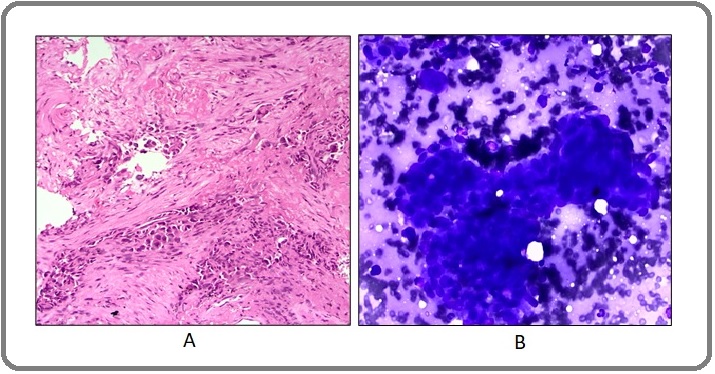
The patient received multiple cycles of chemotherapy (cisplatin and etoposide) and concurrent radiotherapy. A second biopsy was performed from the right lung mass in 2018 and EGFR genotyping by real time PCR showed L858R mutation. Immunohistochemistry for ALK rearrangement was negative. Anti-EGFR tyrosine kinase inhibitor (gefitinib) was initiated and administered for few months during which chemotherapy was withheld. Disease progression was noted in a follow-up PET-CT scan in 2019, showing soft tissue and left adrenal lesions (Figure 1B), which prompted re-start of chemotherapy with cisplatin and etoposide and subsequently changed to pemetrexed based regimen. A third PET-CT scan in 2021 showed multiple metastatic lesions in the liver, adrenal and subcutaneous soft tissue masses on the arms and upper back. Fine needle aspiration was performed from the subcutaneous lesion on the back (Figure 2B) and EGFR and ALK were tested in the cytological material by real time PCR. This revealed ALK-EML4 rearrangement and EGFR wild type. Based on this finding, crizotinib was initiated and the radiological response was assessed by a follow-up PET scan after few months. The scan showed an interesting and dramatic response which included a decrease in size of the primary lung lesion (SUV 4.1),
complete regression of mediastinal nodes, decrease in the size of the metastatic lesions in liver and adrenal gland (Figure 1C). There was a remarkable improvement in the overall clinical profile of the patient which reflected in his significantly improved general condition.
Discussion
Intratumoral heterogeneity in lung adenocarcinoma has been extensively demonstrated by various studies, establishing the dynamic molecular profile of these tumours with multiple spatially or temporally heterogenous clones. Molecular heterogeneity is reflected by morphological heterogeneity with non-EGFR mutations dominating in poorly differentiated areas [3-5]. It is also known that more than one driver mutation may be present in a single tumour with multiple branching or private mutations coexisting with the dominant clones, the latter being strongly associated with development of secondary resistance to targeted therapy [13, 14]. This was further elucidated by Pelsoi et al, in a cohort of 20 cases of lung adenocarcinoma, demonstrating more than one mutation in multiple samples from different areas of a single tumour in nearly 60% of cases and mutations other than EGFR prevailing in poorly differentiated areas of the tumour. In addition, they also established branching ALK and KRAS mutations in EGFR dominant tumours and intriguingly observed the co-occurrence of exon 18 rare EGFR mutations over the more common exon 19 and 21 mutations in ALK rearranged and KRAS mutated tumours. They postulated that that “mutations function dynamically in pulmonary ADC depending on stochastic combinations of gene aberrations, their interaction and order of appearance” [15]. Further, sub-clones contribute to poor differentiation, disease progression, metastasis and a grim prognosis [3, 8].
Our case is an apt demonstration of the established literature, where EGFR mutation and ALK rearrangement co-existed in the same tumour which was previously classified as wild-type. Further, it was the ALK clone that dominated in the metastatic sites, probably owing to its association with more aggressive disease [16]. Further, ALK appears to be the driver mutation with EGFR (exon 21 point mutation L858R) being a branching mutation or minor subclone evidenced by the fact that ALK-targeted therapy (crizotinib) caused a shrinkage of the primary tumour mass in addition to regression in the metastatic disease. A similar scenario was reported by Wu et al, demonstrating two cases with ALK and ROS1 alterations in metastatic lung adenocarcinoma which presented as breast nodules and showed a significant regression in both primary and metastatic tumours with crizotinib [17].
Livi et al, evaluated the efficacy of percutaneous ultrasound-assisted needle aspiration/biopsy for “superficial metastasis” such as subcutaneous soft tissue, muscle, lymph nodes etc., for diagnosis and molecular profiling and found this method to be effective, safe and affordable in addition to an excellent diagnostic yield for ancillary testing (immunohistochemistry and molecular testing) [18]. This is significant in cases undergoing re-biopsy to assess progression of disease and evaluate resistance to targeted therapy, especially in patients with sub-optimal clinical conditions, or unfavourable tumour location [19, 20].
In conclusion, molecular testing from accessible metastatic sites in lung cancer is recommended, which can be crucial for deciding the dominant mutation and hence initiate the appropriate targeted therapy. Awareness of intratumoral molecular heterogeneity in lung cancer and multiple-site sampling will ensure a more complete assessment of prognosis and therapeutic options in recurrent and metastatic disease.
Disclosure
This case report did not receive any specific grant from funding agencies in the public, commercial, or not- for-profit sectors.
Acknowledgments
Statement of Transparency and Principals:
· Author declares no conflict of interest
· Study was approved by Research Ethic Committee of author affiliated Institute.
· Study’s data is available upon a reasonable request.
· All authors have contributed to implementation of this research.
References
- Precision Medicine in Lung Cancer Treatment Shah DR , Masters GA . Surgical Oncology Clinics of North America.2020;29(1). CrossRef
- International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma Travis WD , Brambilla E, Noguchi M, Nicholson AG , Geisinger KR , Yatabe Y, Beer DG , et al . Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2011;6(2). CrossRef
- Intratumor heterogeneity in localized lung adenocarcinomas delineated by multiregion sequencing Zhang J, Fujimoto J, Zhang J, Wedge DC , Song X, Zhang J, Seth S, et al . Science (New York, N.Y.).2014;346(6206). CrossRef
- In situ mutation detection and visualization of intratumor heterogeneity for cancer research and diagnostics Grundberg I, Kiflemariam S, Mignardi M, Imgenberg-Kreuz J, Edlund K, Micke P, Sundström M, Sjöblom T, Botling J, Nilsson M. Oncotarget.2013;4(12). CrossRef
- Cancer. Attack of the clones Govindan R. Science (New York, N.Y.).2014;346(6206). CrossRef
- Genotypic and Histological Evolution of Lung Cancers Acquiring Resistance to EGFR Inhibitors Sequist LV , Waltman BA , Dias-Santagata D, Digumarthy S, Turke AB , Fidias P, Bergethon K, et al . Science translational medicine.2011;3(75). CrossRef
- EML4-ALK mutations in lung cancer that confer resistance to ALK inhibitors Choi YL , Soda M, Yamashita Y, Ueno T, Takashima J, Nakajima T, Yatabe Y, et al . The New England Journal of Medicine.2010;363(18). CrossRef
- Dissecting Pulmonary Large-Cell Carcinoma by Targeted Next Generation Sequencing of Several Cancer Genes Pushes Genotypic-Phenotypic Correlations to Emerge Pelosi G, Fabbri A, Papotti M, Rossi G, Cavazza A, Righi L, Tamborini E, et al . Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2015;10(11). CrossRef
- High incidence of EGFR mutations in Korean men smokers with no intratumoral heterogeneity of lung adenocarcinomas: correlation with histologic subtypes, EGFR/TTF-1 expressions, and clinical features Sun P, Seol H, Lee H, Yoo SB , Kim H, Xu X, Jheon S, et al . Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2012;7(2). CrossRef
- Single-cell mRNA sequencing identifies subclonal heterogeneity in anti-cancer drug responses of lung adenocarcinoma cells Kim K, Lee HW , Lee H, Kim SV , Seo YJ , Chung W, Eum HH , et al . Genome Biology.2015;16(1). CrossRef
- Molecular testing for selection of patients with lung cancer for epidermal growth factor receptor and anaplastic lymphoma kinase tyrosine kinase inhibitors: American Society of Clinical Oncology endorsement of the College of American Pathologists/International Association for the study of lung cancer/association for molecular pathology guideline Leighl NB , Rekhtman N, Biermann WA , Huang J, Mino-Kenudson M, Ramalingam SS , West H, Whitlock S, Somerfield MR . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2014;32(32). CrossRef
- Association of IASLC/ATS/ERS Histologic Subtypes of Lung Adenocarcinoma With Epidermal Growth Factor Receptor Mutations in 320 Resected Cases Nakamura H, Saji H, Shinmyo T, Tagaya R, Kurimoto N, Koizumi H, Takagi M. Clinical Lung Cancer.2015;16(3). CrossRef
- Using multiplexed assays of oncogenic drivers in lung cancers to select targeted drugs Kris MG , Johnson BE , Berry LD , Kwiatkowski DJ , Iafrate AJ , Wistuba II , Varella-Garcia M, et al . JAMA.2014;311(19). CrossRef
- Clinical cancer genomics: how soon is now? Taylor BS , Ladanyi M. The Journal of Pathology.2011;223(2). CrossRef
- Deciphering intra-tumor heterogeneity of lung adenocarcinoma confirms that dominant, branching, and private gene mutations occur within individual tumor nodules Pelosi G, Pellegrinelli A, Fabbri A, Tamborini E, Perrone F, Settanni G, Busico A, et al . Virchows Archiv: An International Journal of Pathology.2016;468(6). CrossRef
- ALK in lung cancer: past, present, and future Shaw AT , Engelman JA . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2013;31(8). CrossRef
- ALK or ROS1-rearranged breast metastasis from lung adenocarcinoma: a report of 2 cases Wu X, Wang H, Fang M, Li C, Zeng Y, Wang K. Tumori.2019;105(6). CrossRef
- Diagnosis and Molecular Profiling of Lung Cancer by Percutaneous Ultrasound-Guided Biopsy of Superficial Metastatic Sites Is Safe and Highly Effective Livi V, Paioli D, Cancellieri Al, Betti S, Natali F, Ferrari M, Fiorentino M, Trisolini R. Respiration; International Review of Thoracic Diseases.2021;100(6). CrossRef
- The International Association for the Study of Lung Cancer Global Survey on Molecular Testing in Lung Cancer Smeltzer MP , Wynes MW , Lantuejoul S, Soo R, Ramalingam SS , Varella-Garcia M, Meadows Taylor M, et al . Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2020;15(9). CrossRef
- Molecular Testing in Lung Cancer: Still Big Gap in Implementation for Real-World Use Ahn M. Journal of Thoracic Oncology: Official Publication of the International Association for the Study of Lung Cancer.2020;15(9). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2024
Author Details