A Rare Case of Plasma Cell Leukemia: Diagnostic and Prognostic Challenges
Download
Abstract
Introduction: Plasma cell leukaemia (PCL) is an uncommon and aggressive variant of Plasma cell neoplasm characterized by the presence of clonal plasma cells constituting more than 5% of the peripheral blood. PCL is more aggressive and less common than MM. While MM is primarily confined to the bone marrow, PCL often presents with extensive extramedullary involvement and higher circulating plasma cell counts. The prognosis of PCL is significantly worse than MM, with a median survival often less than a year despite novel therapies.
Case presentation: A 59-year-old seropositive (HbsAg positive) male patient, an operated case of Solitary Plasmacytoma, was later diagnosed with PCL given the presence of leucocytosis with 26% atypical plasma cells in the peripheral blood. The patient was then advised of further investigations such as serum protein electrophoresis, immunofixation studies, and Fluorescence in-situ hybridization (FISH), following which the patient was categorised as High-risk category according to Revised International Staging System for multiple myeloma (R-ISS).
Conclusion: This case report describes the difficulties in the diagnostic aspect of the entity and highlights the importance of appropriate morphological diagnosis for a better molecular prognostication.
Introduction
PCL has been defined by the presence of clonal plasma cells constituting more than 5% of the peripheral blood. This condition is exceptionally rare, comprising only 0.6%-4% of all plasma cell neoplasms, with reported occurrences of less than one in a million individuals [1, 2] PCL and MM share a common origin in malignant plasma cells, but their pathophysiology diverges significantly, making PCL a more aggressive and disseminated disease. PCL exhibits a higher degree of plasma cell detachment from the bone marrow microenvironment due to reduced expression of adhesion molecules like CD56. This leads to extensive hematogenous spread and extramedullary involvement in organs such as the liver and spleen, which is rare in MM [3].
Unlike MM, PCL cells display a more autonomous proliferative capacity, evidenced by higher mitotic activity and increased expression of proliferative markers. Additionally, PCL is associated with a more complex and unstable genomic landscape, including TP53 mutations and deletions of chromosome 17p, which contribute to impaired DNA damage response and unchecked cell cycle progression. MYC rearrangements, which are also more common in PCL, drive further oncogenic transformation, enhancing cell survival and proliferation [3].
PCL has a poorer prognosis compared to MM, with a median overall survival (OS) below one year [3].
Because of the relatively low incidence of PCL, our case report provides a valuable opportunity to explore its clinical and pathological features, along with various treatment approaches and their outcomes.
Case report Chief complaints
A 59-year-old male complained of fever, cough, and bilateral leg pain, which started one month before admission and got aggravated in the past 5 days.
History of past illness
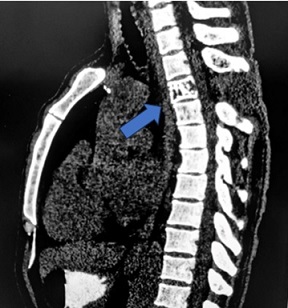
The patient was seropositive (HBsAg reactive) and had a previous history of weakness and tingling in both lower limbs. He was a known case of Solitary plasmacytoma with past radiotherapy and was operated on for intradural lesion at D3 level. CT scan reported a lytic expansile lesion in D3 vertebral body and transverse process (Figure 1).
Figure 1. CT Scan Shows a Lytic Expansile Lesion with Cortical Thinning and Coarse Bony Trabeculae.
Physical examination
On admission, vital signs of the patient were within normal limits, and there were no abnormalities on physical examination.
Laboratory evaluation
Further laboratory workup was done for the patient, the results of which are presented in Table 1.
| RBC | Rouleaux formation seen |
| WBC | Neutrophils-56, Eosinophils-01, Basophils-00, |
| Lymphocytes-10, Monocytes-07. | |
| 26% atypical plasma cells are noted. | |
| Platelets | Mild thrombocytopenia, few platelet clumps noted. |
The laboratory parameters done for the patient, such as serum proteins, liver function tests, HIV and HCV antibodies, urine routine examination, and clinical chemistry (sodium, potassium, chloride, urea, creatinine) came out as normal. Complete blood counts revealed leukocytosis (WBC: 14,000/µL) with an elevated neutrophil count reflecting an ongoing inflammatory or neoplastic process. Mild thrombocytopenia and elevated inflammatory markers, including CRP (206 mg/L) and ESR (92 mm/hr), further supported an underlying aggressive disease.
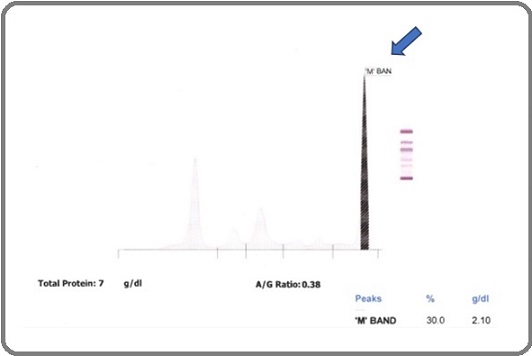
The patient’s serum protein electrophoresis (Table 2, Figure 2) and immunofixation studies (Table 3) confirmed IgG kappa monoclonal protein presence, corroborated by markedly elevated IgG and an extreme free kappa/ lambda ratio (Table 4) Hypoalbuminemia and significantly elevated beta-2 microglobulin (Table 5) indicated a high tumour burden and poor prognosis.
| Parameter | Patient’s value (gm%) | Biological Reference Interval (gm%) |
| Albumin | 1.81 | 3.50 – 5.60 |
| Alpha 1 | 0.58 | 0.10 – 0.30 |
| Alpha 2 | 1.11 | 0.20-1.00 |
| Beta globulins | 0.61 | 0.50-1.10 |
| Gamma globulins | 2.70 | 0.50-1.50 |
Figure 2. Serum Protein Electrophoresis.
| Immunoglobulins | Observed Band |
| IgG | Present |
| IgA | Absent |
| IgM | Absent |
| Kappa | Present |
| Lambda | Absent |
| M band | Present |
| Test | Observed value | Biological Reference Interval |
| Free kappa (light chain) | 620 | 3.30 to 19.4 mg/L |
| Free lambda (light chain) | 19 | 5.71 to 26.30 mg/L |
| Free kappa/ free lambda ratio | 32.63 | 0.26 to 1.65 |
| Test | Observed value | Biological Reference Interval |
| IgG level | 3270 | 700-1600 mg/dL |
| IgA level | 173 | 70-400 mg/dL |
| IgM level | 55.4 | 40-230 mg/dL |
| Beta-2 microglobulin | 6882 ng/ml | 609 – 2164 ng/ml |
sss
The presence of reactive HBsAg, abnormal liver function tests (AST: 115 U/L, conjugated bilirubin: 0.63 mg/dL), and low calcium (7.70 mg/dL) raised concerns for liver dysfunction and potential disease-related complications.
Further diagnostic work-up
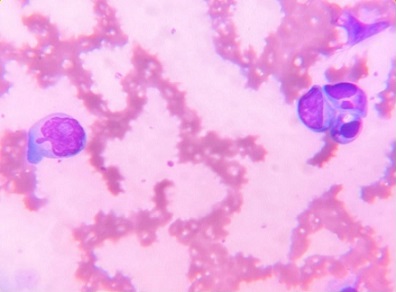
On peripheral blood smear, 26% atypical plasma cells were noted, along with rouleaux formation, suggesting increased circulating monoclonal proteins (Table 1 and Figure 3).
Figure 3. Peripheral Blood Smear Showing Atypical Immature Plasma Cells.
On bone marrow examination, aspiration findings showed adequate marrow particles. The marrow was hypercellular with relatively suppressed erythroid and myeloid series and adequate megakaryocyte series.
About 85% of immature and mature plasma cells.Plasmablasts were also seen with occasional binucleated and trinucleated forms (Figure 4a).
Figure 4. Bone Marrow Evaluation. 4a, Bone marrow aspirate shows plasmablasts; 4b, Bone marrow biopsy: immature and mature plasma cells along the intertrabecular and paratrabecular spaces (40x). The inset shows one such intertrabecular space. (10x); 4c, Reticulin stain shows Grade II marrow fibrosis.
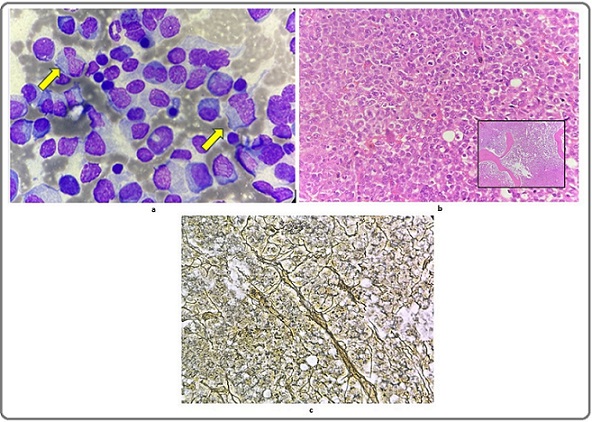
The bone marrow biopsy (Figure 4b) was done along with Perls stain, which revealed normal bone marrow iron stores (Grade III) and reticulin stain, which showed Grade II marrow fibrosis (Figure 4c).
Following this, the patient was advised to undergo immunophenotyping and a molecular panel for plasma cell neoplasm. Cytogenetic analysis by FISH was positive for 3 copies of chromosome 11, deletion 17p and 13q, and negative for del (11q), trisomy of chromosome 12, and 1p Deletion/1q Amplification in all the cells analysed. Given the presence of del (17p), the case was classified under the High-risk category according to R-ISS. Table 6 enlists the risk stratification in plasma cell myeloma according to the latest WHO guidelines [2].
| Risk model | Low | Standard | High |
| ISS | I: Serum B2M < 3.5mg/L, serum albumin ≥3.5g/dL | II: Not stage I or III | III: Serum B2M ≥ 5.5 mg/L |
| IMWG | ISS I/II, age < 55 years, and absence of all of t (4;14), del (17p13), and 1q21+ | Not low or high risk | ISS II/III and t (4;14) or del (17p13) |
| R-ISS | I: ISS I and absence of all of del (17p13), t (4;14), and t (14;16) | II: Not R-ISS I or III | III: ISS III with either high serum LDH or presence of any of del (17p13), t (4;14), or t (14;16) |
IMWG, International Myeloma Working Group; ISS, International Staging System; R-ISS, Revised International Staging System.
Final diagnosis
The collective hematological, radiological, and biochemical findings were suggestive of PCL.
Treatment
We lost follow-up with the patient. However, he was treated with myeloma-directed therapy at a different institute, the details of which were not available to us.
Discussion
Plasma cell neoplasms originate from monoclonal mature B-cells that have undergone terminal differentiation, and their distinguishing feature lies in the dominance and proliferation of plasma cells devoid of a naïve B-cell component. The accurate diagnosis and clinical management of plasma cell neoplasms require the comprehensive integration of laboratory parameters, along with morphology, immunophenotype, and cytogenetics [4, 5].
Recognizing PCL as a potential diagnosis alongside suspected multiple myeloma (MM) becomes crucial [2]. While MM frequently presents with osteolytic bone lesions, these are less common in PCL. Instead, PCL is associated with an increase in lactate dehydrogenase (LDH) levels, severe cytopenia, and a risk of tumor lysis syndrome at the initiation of treatment. Another key clinical distinction is the higher prevalence of light chain-only and non-secretory disease in PCL compared to MM, which predominantly exhibits IgG or IgA monoclonal protein production [3].
Diagnosis of PCL traditionally requires ≥ 5% circulating clonal plasma cells in peripheral blood (Table 7) [4], MM is diagnosed based on clonal plasma cells in the bone marrow and myeloma-defining events, CRAB criteria (hypercalcemia, renal failure, anemia, and bone lesions) [3].
| Smouldering multiple myeloma |
| Essential: Serum monoclonal protein (IgG or IgA) ≥ 3 g/dL, or urinary monoclonal protein ≥ 500 mg per 24 h and/or clonal bone marrow plasma cells 10–60% |
| AND: Absence of myeloma-defining events or amyloidosis |
| Multiple myeloma |
| Essential: Clonal bone marrow plasma cells ≥ 10% or biopsy-proven bony or extramedullary plasmacytoma |
| AND: Any one or more of the following myeloma-defining events: |
| Evidence of end-organ damage that can be attributed to the underlying plasma cell proliferative disorder, specifically: |
| ·Hypercalcaemia: serum calcium >0.25mmol/L (>1mg/dL) higher than the upper limit of normal or > 2.75 mmol/L (> 11mg/dL) |
| ·Renal insufficiency: creatinine clearance < 40 mL per minute or serum creatinine > 177μmol/L (>2mg/dL) |
| ·Anaemia: haemoglobin value >2g/dL below the lower limit of normal, or a haemoglobin value of <10g/dL |
| Bone lesions: one or more osteolytic lesions on skeletal radiography, CT, or PET-CT OR |
| Clonal bone marrow plasma cell percentage ≥ 60% OR |
| Involved: uninvolved serum free light chain ratio ≥ 100 (involved free light chain level must be ≥ 100 mg/L) OR |
| More than one focal lesion (≥ 5 mm in size) on MRI studies. |
| Plasma cell leukaemia |
| Essential: Presence of ≥ 5% circulating plasma cells in peripheral blood smears in patients otherwise diagnosed with plasma cell myeloma |
A key diagnostic tool in our case study was the peripheral smear examination, which showed 26% plasma cells. Morphological assessment is also crucial for diagnosing PCL and distinguishing it from similar-looking cells like erythroid precursors and osteoblasts (Figure 5a-5f).
Figure 5. Morphological differentials. 5a, 5b, Plasmablast; 5c, Megakaryocyte; 5d, Plasma cell; 5e, Osteoblast; 5f, Intermediate erythroblast [6].
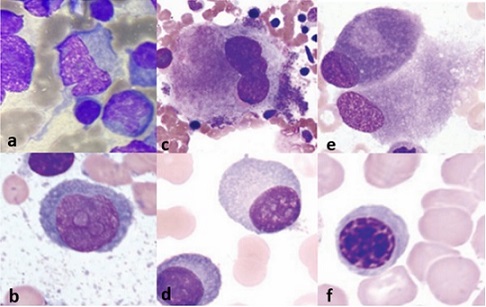
Genetically, PCL exhibits higher rates of TP53 mutations, 17p deletions, and MYC rearrangements, while MM has more frequent KRAS and NRAS mutations [3]. PCL exhibits greater resistance to treatment than MM, mainly due to its reduced dependence on the bone marrow microenvironment. This dissemination is associated with the loss of key adhesion molecules [CD56, LFA- 1, and very late antigen-5 (VLA-5)], which contribute to immune evasion by preventing effective recognition and elimination by T cells and natural killer (NK) cells. Additionally, PCL cells downregulate antigen-presenting HLA class I molecules, further impairing immune surveillance and allowing tumor cells to evade destruction. These factors collectively enable PCL cells to survive outside the bone marrow, resist standard myeloma therapies, and spread aggressively throughout the body [6].
Key survival determinants in PCL include tumor stage, size, histological type, vascular invasion, metastasis, liver function, AFP levels, and treatment modality. Early-stage, well-differentiated tumors without vascular invasion or metastases, along with good liver function and low AFP levels, indicate a better prognosis. In contrast, advanced-stage, poorly differentiated tumors with vascular invasion, extrahepatic spread, liver failure or cirrhosis, and high AFP levels are linked to worse outcomes. Surgical resection and liver transplantation improve survival, while inoperable cases or palliative treatments are associated with a poorer prognosis [7].
Novel treatments for relapsed/refractory PCL include various targeted therapies that aim to improve survival outcomes. Monoclonal antibodies such as rituximab, elotuzumab, and daratumumab have shown promise in treating PCL by targeting specific surface antigens on plasma cells. Additionally, second-generation proteasome inhibitors and immunomodulatory drugs, along with inhibitors of histone deacetylase, Akt, and mTOR, are being investigated to enhance treatment efficacy [6].
Chimeric Antigen Receptor T (CAR-T) cell therapy works by engineering T cells to express a receptor that recognizes B-cell maturation antigen, enabling them to selectively bind and kill leukemic plasma cells. In the context of relapsed/refractory PCL. CAR-T therapy has emerged as a promising treatment option, particularly targeting B-cell maturation antigen (BCMA), which is highly expressed on malignant plasma cells [8].
TP53 mutations are rare at the initial diagnosis of MM and typically indicate disease progression. Despite its relatively low incidence early on and its characterization as a secondary event in MM pathogenesis, del (17p) remains among the most clinically significant chromosomal abnormalities. Its detection categorizes patients into a high-risk category in the IMWG risk model (Table 6) [9]. However, the cost and specialized requirements of FISH pose limitations in its widespread application, necessitating more accessible diagnostic alternatives for comprehensive patient diagnosis.
In conclusion, plasma cell leukaemia (PCL) represents the most aggressive type of monoclonal gammopathy, a rare disorder characterized by abnormal plasma cell growth. With the average age of onset between 52 and 65 years, approximately a decade earlier than MM, diagnosticians must maintain a high index of suspicion, especially when encountering similar symptoms in relatively younger patients. By remaining attentive to these nuances, diagnosticians can effectively navigate the challenges associated with the diagnosis of PCL and provide optimal care for affected individuals. PCL and MM are distinct conditions with unique clinical and pathological features, necessitating different treatment approaches. Furthermore, this case report emphasizes the importance of accurate morphological interpretation and thorough diagnostic evaluation to ensure timely and accurate diagnosis, thereby facilitating prompt initiation of appropriate management strategies and optimizing patient outcomes. To enhance our understanding and management of PCL, it’s imperative to conduct collaborative research efforts aimed at establishing precise diagnostic criteria and ensuring appropriate treatment interventions for this condition.
Statement of ethics
The authors attest that this case report was determined to not require the Institutional Review Board/Ethics Committee review, and the corresponding protocol/ approval number is not applicable. The authors certify that they have obtained appropriate patient consent. The patient has given the consent for the clinical information to be reported in the journal.
Declaration of patient consent
The authors certify that they have obtained all appropriate patient consent forms. In the form, the patient has given his consent for his images and other clinical information to be reported in the journal. The patient understands that his name and initials will not be published and due efforts will be made to conceal his identity, but anonymity cannot be guaranteed.
Data availability statement
All data generated or analysed during this study are included in this article. Further inquiries can be directed to the corresponding author.
Author contribution
NK designed the study, conducted research and organised data. RT wrote initial and final draft of the article. SD and KH provided research material. CRG analysed and interpreted the data. All authors have critically reviewed and approved the final draft of the manuscript.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- Bone marrow pathology Bain BJ , Clark DM , Wilkins BS . John Wiley & Sons; 2019 Apr 15..
- Plasma cell leukemia - one in a million: A case report Jain AG , Faisal-Uddin M, Khan AK , Wazir M, Shen Q, Manoucheri M. World Journal of Clinical Oncology.2019;10(3). CrossRef
- Plasma Cell Leukemia: Definition, Presentation, and Treatment Gundesen MT , Lund T, Moeller HEH , Abildgaard N. Current Oncology Reports.2019;21(1). CrossRef
- The WHO Classification of Haematolymphoid Tumours Cree IA . Leukemia.2022;36(7). CrossRef
- Plasma cell neoplasms and related entities-evolution in diagnosis and classification Fend F, Dogan A, Cook JR . Virchows Archiv: An International Journal of Pathology.2023;482(1). CrossRef
- How I treat plasma cell leukemia Donk NWCJ , Lokhorst HM , Anderson KC , Richardson PG . Blood.2012;120(12). CrossRef
- Prognostic impact of circulating plasma cells in patients with multiple myeloma: implications for plasma cell leukemia definition Granell M, Calvo X, Garcia-Guiñón A, Escoda L, Abella E, Martínez CM , Teixidó M, et al . Haematologica.2017;102(6). CrossRef
- Case report: Plasma cell leukemia secondary to multiple myeloma successfully treated with anti-BCMA CAR-T cell therapy Deng J, Lin Y, Zhao D, Tong C, Chang AH , Chen W, Gao W. Frontiers in Oncology.2022;12. CrossRef
- Molecular spectrum of TP53 mutations in plasma cell dyscrasias by next generation sequencing: an Italian cohort study and overview of the literature Lionetti M, Barbieri M, Manzoni M, Fabris S, Bandini C, Todoerti K, Nozza F, et al . Oncotarget.2016;7(16). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details