The Relationship between Microsatellite Instability and KRAS Mutations in Liver-metastatic Colorectal Cancer: A Preliminary Cross-sectional Study
Download
Abstract
Background: Colorectal cancer (CRC) has a high mortality rate due to the development of liver metastases. Mutations in RAS and mismatch repair (MMR) genes are common in CRC, with Kirsten rat sarcoma viral oncogene (KRAS) mutations occurring in approximately 44% of cases and MSI in 15%. Both mutations are associated with poor prognosis. The study aims to identify MSI status and KRAS mutations in liver-metastatic CRC at a hospital in eastern Indonesia.
Methods: In this cross-sectional study, 57 patients with liver-metastatic CRC were included. We evaluated KRAS mutations and microsatellite instability (MSI) status in patients’ DNA extracted from paraffin blocks. The procedures involved included specimen examination, DNA extraction, and genetic sequencing. The data were analyzed using SPSS version 25.0. Fisher’s exact test was utilized to evaluate the relationship between MSI status and KRAS mutations. A significance level of p<0.05 was considered statistically significant.
Results: This study included patients aged 16–80 years with liver-metastatic colon cancer. Patients were primarily male with left-sided tumors of adenocarcinomatous histopathology and high histopathological grade. Of the 57 subjects, 31.6% had MSI-high (MSI-H) tumors and 21.1% expressed mutant KRAS. The majority of MSI-H tumors (82% of patients) expressed mutant KRAS, while most MSI-low (MSI-L) tumors (60% of patients) expressed wild-type KRAS. However, Fisher’s exact test indicated no significant relationship between MSI status and KRAS mutation status in liver-metastatic colon cancer (p = 0.489).
Conclusions: This study found no significant relationship between MSI status and KRAS mutation status in patients with liver-metastatic colon cancer.
Introduction
The high mortality rate of colorectal cancer (CRC) is generally caused by the development of metastasis during the course of the disease [1, 2]; the liver is a frequent target organ for metastatic spread [3]. Approximately 25% of CRC patients have hepatic metastases at the time of diagnosis, and about 50% of CRC patients will develop liver-metastatic lesions during the course of their disease [4, 5]. If left untreated, patients with liver-metastatic CRC have a median survival of only six to nine months [6].
Mutations in Rat Sarcoma Viral Oncogene Homolog (RAS) and mismatch repair (MMR) genes are the most commonly observed prognostic markers in CRC. Among RAS mutations, Kirsten rat sarcoma viral oncogene (KRAS) mutations (85%) are the most frequently found, followed by neuroblastoma ras viral oncogene homolog (15%) and Harvey Rat sarcoma viral oncogene homolog HRAS (1%). KRAS mutations occur in approximately 44% of CRC cases; the majority of mutations are located in codons 12 and 13 of exon 2 (80% are G12D, G12V, G12C, G12A, or G13D mutations) [7]. MMR gene mutations or microsatellite instability (MSI) are found in 15% of CRC patients, 12% of which are sporadic CRC cases (the remaining 3% are nonpolyposis hereditary CRC cases) [8]. Meta-analyses have reported that KRAS mutations are found in 25–52% of patients with liver- metastatic colon cancer. Most studies report that KRAS mutations are negative prognostic factors for overall and disease-free survival [9]. KRAS mutations are known to be associated with increased heat shock protein expression and activation of PI3K/mTORC2-dependent AKT, thus enhancing cancer cell survival in the liver [10]. MSI-high (MSI-H) tumors are identified in approximately 15% of CRC patients. Deficiencies in DNA mismatch repair protein such MutS homolog 2, MutL protein homolog 1, and mutS homolog 6 protein production can result in impaired mismatch base detection, leading to the expression of abnormal proteins that can be recognized as neoantigens by the immune system. Additionally, this deficiency causes apoptosis to fail after DNA damage is detected. The MMR-deficient/MSI-H phenotype has been associated with the inefficacy of fluoropyrimidine therapy in patients with stage II or III CRC [11]. Interestingly, the proportion of MSI-H tumors among patients with liver-metastatic colon cancer has not been reported to date.
The ability to identify genomic variants has revolutionized clinicians’ understanding of how cancer develops. Molecular cancer biomarkers are indicators that can be detected in every patient and can help estimate cancer risk, cancer incidence, patient prognosis, and response to therapy. Our institution is a central referral hospital in eastern Indonesia, where no previous research has been conducted to determine MSI status and identify KRAS mutations in liver-metastatic CRC. Therefore, this study aimed to identify and understand the relationship between MSI status and the presence of KRAS mutations in liver-metastatic CRC at our institution.
Materials and Methods
This cross-sectional study was conducted at Wahidin Sudirohusodo Hospital and Hasanuddin University Hospital, Makassar, Indonesia. Patient data were collected from 2021 to 2023. The study sample included all patients with liver-metastatic colon cancer. The estimated number of participants was calculated using Lemeshow’s formula [12-14]; the minimum sample size for each group (case and control) was 25 subjects. A consecutive sampling technique was applied in this study.
Liver-metastatic colon cancer
Metastases that were detected at the initial diagnosis of colon cancer (de novo) were categorized as liver-metastatic colon cancer. Colon cancer patients who presented for the first time with radiological signs and clinical symptoms of liver metastasis underwent assessment through abdominal computerized tomography scan, abdominal ultrasound, and abdominal magnetic resonance imaging.
KRAS mutations
KRAS mutations were detected by extracting DNA from paraffin blocks and performing a polymerase chain reaction (PCR). Results are provided on a binary scale (wild-type or mutant).
MSI status
MSI was also detected by extracting DNA from paraffin blocks and performing PCR, with results reported on a binary scale (MSI-low [MSI-L] or MSI-H).
Research procedure
Patients with right or left colon cancer who seek treatment at the Digestive Surgery Department have paraffin blocks collected from biopsy/surgical specimens at the time of initial diagnosis. Subsequently, examination of MSI status and KRAS expression is performed.
Patient Selection
The study included patients with a confirmed anatomic pathological diagnosis of liver-metastatic colon cancer who underwent colon resection. The inclusion criteria were: patients with liver-metastatic colon cancer and resectable liver metastases (defined as having a limited number of metastases, typically up to 3–4 lesions, metastases located in areas of the liver that can be safely resected, and smaller lesions [<5 cm]), absence of extrahepatic metastases, a positive response to neoadjuvant therapy, and a Karnofsky performance status score above 70. Complete clinical data were required for inclusion, and patients with damaged or insufficient paraffin blocks, or those with malignancies in other organs, were excluded.
Specimen Preparation
Paraffin-embedded sections from right or left colon cancer tumors, as well as from liver metastases, were stained with hematoxylin and eosin to assess whether the blocks were suitable for analysis. The paraffin blocks were then sectioned into 10 µm thick slices, which were deparaffinized by soaking in xylene for 30 minutes, followed by treatment with alcohol.
Microdissection and Incubation
After deparaffinization, the tumor tissue was microdissected from the slides. The microdissected tissue was incubated overnight with proteinase K at 55°C to prepare it for further analysis.
DNA extraction procedure and PCR
DNA was extracted from the prepared tissue using the QIAamp DNA FFPE Tissue Kit (QIAGEN). The quality of the extracted DNA was assessed using absorbance ratios (A260/A280 and A260/A230) to ensure it met the criteria for genetic sequencing.
Genetic Analysis
Once the DNA quality was confirmed, genetic sequencing was performed using the AmpliSeq Cancer Hotspot panel v2 for Illumina. This included evaluating KRAS mutations at codons 12 and 13 through PCR and agarose gel electrophoresis.
Data analysis
Data in this study were collected in tabular form using SPSS version 25.0 (Armonk, NY; IBM Corp.). Univariate and bivariate analyses were conducted. To assess the relationship between MSI status and KRAS mutation status, Fisher’s exact test was used. A p-value of less than 0.05 was considered statistically significant.
Results
Patient characteristics
Patients with liver-metastatic colon cancer were 16-80 years old and primarily male. Tumors were predominantly located on the left side, of adenocarcinomatous histopathology, and of high histopathological grade (Table 1).
| Variable | MSI status | KRAS mutation status | ||||
| MSI-low | MSI-high | p-value | Wild-type | Mutant | p-value | |
| (n, %) | (n, %) | (n, %) | (n, %) | |||
| Age (years) | 55.8 ± 11.8 | 51.4 ± 15.7 | 53.3 ± 14.8 | 47.9 ± 15.0 | ||
| 16–25 | 1 (14) | 0 (0) | 0.386 | 1 (2.3) | 0 (0) | 0.406 |
| 26–35 | 6 (18) | 1 (14.3) | 4 (9.3) | 3 (42.86) | ||
| 36–45 | 8 (12) | 2 (20) | 10 (23.26) | 0 (0) | ||
| 46–55 | 7 (58.3) | 5 (41.7) | 8 (18.6) | 1 (14.28) | ||
| 56–65 | 10 (76.9) | 3 (23.1) | 9 (20.93) | 3 (42.86) | ||
| >65 | 7 (50) | 7 (50) | 11 (25.8) | 0 (0) | ||
| Sex | ||||||
| Male | 8 (25.8) | 23 (74.2) | 0.394 | 23 (74.2) | 8 (25.8) | 0.516 |
| Female | 10 (38.5) | 16 (61.5) | 22 (84.6) | 4 (15.4) | ||
| Location | ||||||
| Right | 9 (33.3) | 18 (66.7) | 0.787 | 25 (92.6) | 2 (7.4) | 0.23 |
| Left | 9 (30.0) | 21 (70.0) | 20 (66.7) | 10 (33.3) | ||
| Histopathological type | ||||||
| Adenocarcinoma | 11 (31.4) | 24 (68.6) | 0.995 | 29 (82.9) | 6 (17.1) | 0.602 |
| Mucinous | 5 (31.2) | 11 (68.8) | 12 (75.0) | 4 (25.0) | ||
| Signet ring cell carcinoma | 2 (33.3) | 4 (66.7) | 4 (66.7) | 2 (33.3) | ||
| Histopathological grade | ||||||
| High | 8 (44.4) | 10 (55.6) | 0.178 | 15 (83.3) | 3 (16.7) | 0.539 |
| Moderate | 1 (11.1) | 8 (88.9) | 7 (77.8) | 2 (22.2) | ||
| Low | 1 (20) | 4 (80.0) | 5 (100) | 0 (0) |
Note: MSI, microsatellite instability; KRAS, Kirsten rat sarcoma viral oncogene.
This study also demonstrated that mutant KRAS is most prevalent in the left colon (sigmoid colon cancer), whereas wild-type KRAS is most prevalent in the left colon (ascending colon cancer). We also found that MSI-L tumors are equally prevalent in sigmoid and ascending colon cancer (30% and 33.3%, respectively), while MSI-H tumors are more prevalent in the left colon (sigmoid colon cancer) at 66.7%.
MSI status of liver-metastatic colon cancer
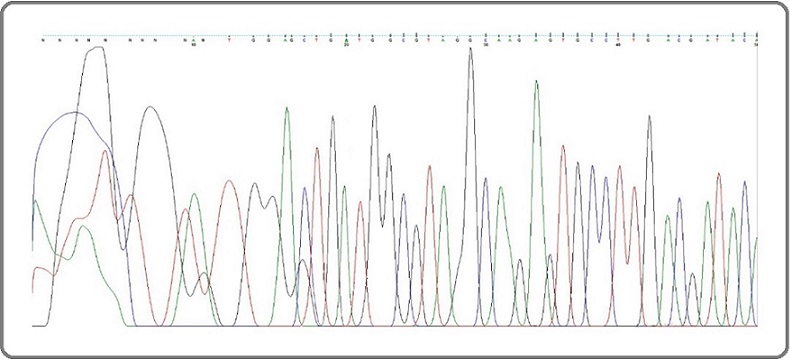
In this study, 18 subjects (31.6%) with liver-metastatic colon cancer had MSI-H tumors (Table 2; Figure 1).
| MSI status | n | % |
| MSI-low | 39 | 68.4 |
| MSI-high | 18 | 31.6 |
Note: MSI, microsatellite instability.
Figure 1. Electropherogram Depicting MSI in Liver-metastatic Colon Cancer.

KRAS expression in liver-metastatic colon cancer
In this study, 45 out of 57 subjects (78.9%) with liver-metastatic colon cancer expressed mutant KRAS (Table 3; Figure 2).
| KRAS expression status | n | % |
| Wild-type | 12 | 21.1 |
| Mutant | 45 | 78.9 |
Note: KRAS, Kirsten rat sarcoma viral oncogene.
Figure 2. Electropherogram of KRAS Expression in Liver-metastatic Colon Cancer.
Relationship between MSI status and KRAS mutations in liver-metastatic colon cancer
In this study, the majority of patients with MSI-H tumors had mutant KRAS (82%) expression. On the other hand, most MSI-L tumors expressed wild-type KRAS (60%). Fisher’s exact test indicated no significant relationship between MSI status and KRAS mutation status in liver-metastatic colon cancer (p = 0.489; Table 4).
| MSI status | Mutant KRAS | Wild-type KRAS | p-value |
| n (%) | n (%) | ||
| MSI-high | 32 (82) | 7 (18) | 0.489 |
| MSI-low | 13 (60) | 5 (40) |
Note: MSI, microsatellite instability; KRAS, Kirsten rat sarcoma viral oncogene.
Discussion
In this study, liver-metastatic colon cancer was more common in men (54.39%) than women (45.61%), and liver-metastatic colon cancer was more common expressed MSI-L and mutant KRAS. These findings are consistent with the Wu et al. study, which found that although the incidence of colon cancer was higher in men (60.2%), women experienced higher rates of extrahepatic metastasis (28.2% vs. 19.8%) [15, 16]. Additionally, Ardito et al. found that liver-metastatic colon cancer more frequently expressed mutant vs. wild-type KRAS (54.20% vs. 45.80%) and was more common in men than women (62.6% vs. 37.4%) [17].
With respect to age, our study demonstrated that the incidence of colon cancer increases with age after age 25. Specifically, there is only one case in the 18-25-year-old age group, but at least seven cases in the 25-and-above age group. Mangi et al. also showed that the prevalence of CRC is higher in individuals aged 25 and above (81.6%) [18]. With respect to cases of liver-metastatic colon cancer, this finding aligns with studies by Ituarte et al. and Xu and Wang, which found the highest incidence in individuals over 44 years old [15, 16]. According to Gao et al., the incidence of liver-metastatic colon cancer in younger patients may be due to high rates of misdiagnosis and relatively rapid disease progression. Additionally, colon cancer in younger individuals is generally moderately to poorly differentiated, making it more likely to metastasize [19].
The data in this study show that mutant KRAS is more prevalent in left-sided colon cancer. This finding is consistent with those of Diener and Fichtner-Feigl, in which the prevalence of mutant KRAS was 30–45% in CRC and 25–52% in liver-metastatic CRC [20]. Additionally, in an analysis of 552 Polish CRC patients, Ciepiela et al. found that there were more cases of left-sided colon cancer (45.2%, p = 0.0002) among liver-metastatic (54.5%) and KRAS-mutant (42.8%) cases. In their analysis, colon cancer was not homogeneous; rather, cancer development was closely related to its location [21]. This finding differs from that of Xie et al., in which mutant KRAS was more common in right-sided colon cancer (p = 0.010) [22]. Specifically, in the study by Xie et al., there were 32 subjects with mutant KRAS out of a total of 37 right-sided colon cancer patients, and 30 subjects with mutant KRAS out of 79 left-sided colon cancer patients [22].
Another finding in this study indicates that MSI-H tumors are equally prevalent in patients with right- and left-sided colon cancer (9 subjects in each group), but MSI-L tumors are more prevalent in patients with left- vs. right-sided colon cancer (70% versus 66.7%). This finding is consistent with that of Han et al., in which MSI-L tumors were more prevalent in left-sided colon cancer cases [23]. MSI-H tumor distribution differs between right- and left-sided colon cancer. Right-sided MSI-H tumors are more prevalent and often exhibit aggressive features, including higher tumor stages and increased mucinous carcinoma types [24]. Right-sided colon cancers show a higher prevalence of the CpG island methylator phenotype (CIMP) and are often hypermutated, leading to a more aggressive tumor behavior [25]. MSI-H tumors are characterized by a deficiency in MMR mechanisms, leading to an accumulation of mutations in microsatellite regions [26, 27]. Furthermore, right-sided tumors are associated with hypermethylation and a hypermutated state, contributing to poorer prognoses [25]. MSI-H tumors often present with distinct clinical features, such as poorly differentiated histology and a bimodal age distribution [26, 28]. In contrast, left-sided colon cancer has a lower prevalence of MSI-H tumors, with only 10-15% of cases showing high levels of microsatellite instability. Left-sided MSI-H tumors tend to have lower tumor stages and less frequent abnormal carcinoembryonic antigen levels [24]. Left-sided colon cancers have less frequent methylation of human mutL homolog-1 and lower CIMP rates, indicating a different pathway for MSI acquisition [29]. This difference in distribution may impact treatment strategies and prognosis for patients with colon cancer, as MSI-H tumors have been shown to respond differently to certain types of chemotherapy and immunotherapy [30, 31]. Understanding the distribution of MSI-H tumors in right- and left-sided colon cancer is important for guiding personalized treatment approaches for patients with this disease. The distribution of MSI-H tumors varies significantly between right- and left-sided colon cancers, reflecting distinct biological and clinical characteristics.
The relationship between MSI and KRAS mutations appears complex, with some studies indicating no statistically significant association. For instance, in a study of CRC patients from Tunisia, no significant associations were found between KRAS mutations and MSI status despite KRAS mutations in 31.5% of cases [29]. Additionally, a large analysis of CRCs showed that while KRAS mutations were common, their prognostic significance varied between MSI and MSS groups, with no significant differences in survival outcomes for MSI patients [32]. Conversely, another study indicated that while KRAS mutations were prevalent in MSI gastric cancer, they were associated with poorer outcomes in microsatellite stable (MSS) cases [33]. Additionally, a large analysis of CRCs showed that while KRAS mutations were common, their prognostic significance varied between MSI and MSS groups, with no significant differences in survival outcomes for MSI patients [34]. This suggests that while KRAS mutations can coexist with MSI, their clinical implications may differ significantly based on the tumor’s MSI status. This study also found that KRAS mutations (17.1%) were more prevalent in adenocarcinomatous tumors (61.4%). This result is consistent with that of Ciepiela et al., in which most tumors (98.9%) were adenocarcinomas, followed by medullary carcinomas (0.2%), neuroendocrine tumors, and goblet cell carcinoid tumors [21]. Alexander et al. also reported a higher proportion of high-grade, MSI-H adenocarcinomatous tumors (38%) [35]. Thus, the findings of this study are consistent with those in the literature in that adenocarcinoma was the most prevalent histopathological type (35%).
MSI and KRAS mutations are critical biomarkers in liver-metastatic CRC, influencing treatment outcomes and survival rates. Studies indicate that MSI-positive tumors exhibit a higher prevalence of KRAS mutations, with 66.7% in MSI-positive versus 52.3% in MSS cases [36]. In a large cohort from the National Cancer Database, the prevalence of MSI-H was 3.1%, while KRAS mutations were found in 42.4% of patients. Notably, geographic discrepancies exist, with higher MSI rates in certain United States regions. Furthermore, KRAS mutations are associated with poorer overall survival, particularly in right-sided CRC [37]. The interaction between these biomarkers is crucial, as they can affect treatment responses, such as resistance to therapies in Raf murine sarcoma viral oncogene homolog B (BRAF)-mutant cases with concurrent KRAS mutations [38]. The lack of association between MSI status and KRAS mutations in liver-metastatic CRC can be attributed to several biological factors. Firstly, studies indicate that KRAS mutations do not significantly influence survival outcomes in patients with synchronous liver metastases, regardless of tumor sidedness [39]. Additionally, while KRAS mutations are linked to poorer recurrence-free survival in left-sided tumors, this association is not observed in right-sided tumors [40]. Furthermore, the interplay between KRAS status and tumor laterality suggests that the biological behavior of tumors may differ based on their location, complicating the relationship with MSI [41]. Lastly, other mutations, such as BRAF, may also interact with KRAS status, further obscuring the association with MSI [42]. These findings highlight the complexity of genetic interactions in CRC, suggesting that the absence of a clear link between MSI and KRAS mutations may reflect underlying biological diversity rather than a straightforward relationship. Conversely, some studies have shown that MSI status can influence treatment responses and outcomes, indicating that while KRAS mutations may not correlate with MSI, they still play a critical role in the overall prognosis of CRC patients with liver metastases [43].
CRC metastasis to the liver exhibits significant genomic diversity, particularly in Asian populations. In a study of Chinese patients, KRAS mutations were found in 42% of colorectal liver metastases, with tumor protein p53 and adenomatous polyposis coli mutations also prevalent [44]. KRAS mutations correlate with poorer overall survival and disease-free survival, especially in KRAS-mutated tumors, where surgical margins do not significantly impact outcomes [45]. Notably, the KRAS A146 mutation is associated with higher tumor burden and inferior survival compared to other variants [46]. Furthermore, the presence of KRAS mutations influences surgical strategies, although the type of resection may not significantly affect survival outcomes in KRAS-mutated cases [47]. These findings underscore the importance of understanding KRAS mutation status in managing CRC metastasis to the liver in Asian populations, particularly regarding personalized treatment approaches and surgical decisions.
This study has several limitations. First, the sample size is low, 57 patients. Second, it is subject to selection bias, given its retrospective nature. Specifically, most cases were diagnosed at advanced stages. Third, some patients may have been lost to follow-up, potentially leading to lost medical records and data. Fourth, statistical methods highlight the need for more robust analyses in future research. Therefore, more research is needed, particularly in prospective studies with larger sample sizes, to clarify the role of gene mutations in CRC prognosis and implications for personalized medicine or targeted therapies.
In conclusion, we conclude that there is no significant relationship between MSI status and KRAS mutation status in liver-metastatic CRC (p = 0.489). We found that 31.6% of tumors were MSI-H and that 78.9% expressed mutant KRAS, with a majority of MSI-H tumors also having mutant KRAS.
Acknowledgments
This research was supported by the Department of Surgery and the Laboratory of Pathological Anatomy at Wahidin Sudirohusodo Hospital, the Laboratory of Pathological Anatomy at Hasanuddin University Hospital, and the Hasanuddin University Medical Research Center (HUMRC) at the Faculty of Medicine, Hasanuddin University, Makassar, Indonesia.
Conflicts of Interest
The authors certify that there is no conflict of interest with any financial organization regarding the material discussed in the manuscript.
Funding
Self-funding
Ethics approval
This study was approved by the Medical Research Ethics Commission of the Medical Faculty of Hasanuddin University (number: 850/UN4.6.4.5.31/PP36/2023). We promised that the participants’ data were anonymized or maintained with confidentiality, the rights or interests of participants were not invaded, and informed consent was taken from all individual participants.
Authors’ contributions
APP: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing.
WS: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Software, Validation, Visualization, Writing – original draft.
JAU: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
AAZ: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing.
MF: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
All authors read and approved the final version of the manuscript.
References
- Which patients are prone to suffer liver metastasis? A review of risk factors of metachronous liver metastasis of colorectal cancer Hao M, Wang K, Ding Y, Li H, Liu Y, Ding L. European Journal of Medical Research.2022;27(1). CrossRef
- Analysis of the prognostic factors affecting 5-year colorectal cancer survival rates in Makassar, Eastern Indonesia: a retrospective cohort study Arsyad A, Lusikooy Re , Syarifuddin E, Warsinggih W, Labeda I, Mappincara M, et al . Gazzetta Medica Italiana Archivio per le Scienze Mediche.2023;182(3). CrossRef
- The Relationship Between Histopathological Grading and Metastasis in Colorectal Carcinoma Patients Minhajat R, Benyamin AF , Miskad UA . Nusantara Medical Science Journal.2020. CrossRef
- Surgical management of colorectal liver metastases: a European perspective Dunne DF , Jones RP , Malik HZ , Fenwick SW , Poston GJ . Hepatic Oncology.2014;1(1). CrossRef
- Survival after liver resection in metastatic colorectal cancer: review and meta-analysis of prognostic factors Kanas GP , Taylor A, Primrose JN , Langeberg WJ , Kelsh MA , Mowat FS , Alexander DD , Choti MA , Poston G. Clinical Epidemiology.2012;4. CrossRef
- Cytoreduction for colorectal metastases: liver, lung, peritoneum, lymph nodes, bone, brain. When does it palliate, prolong survival, and potentially cure? Stewart CL , Warner S, Ito K, Raoof M, Wu GX , Kessler J, Kim JY , Fong Y. Current Problems in Surgery.2018;55(9). CrossRef
- BRAF and KRAS mutations in metastatic colorectal cancer: future perspectives for personalized therapy Li Z, Zhao , Yu L, Wei M. Gastroenterology Report.2020;8(3). CrossRef
- Immunotherapy in colorectal cancer: rationale, challenges and potential Ganesh K, Stadler ZK , Cercek A, Mendelsohn RB , Shia J, Segal NH , Diaz LA . Nature Reviews. Gastroenterology & Hepatology.2019;16(6). CrossRef
- Clinical significance and prognostic relevance of KRAS, BRAF, PI3K and TP53 genetic mutation analysis for resectable and unresectable colorectal liver metastases: A systematic review of the current evidence Tsilimigras DI , Ntanasis-Stathopoulos I, Bagante F, Moris D, Cloyd J, Spartalis E, Pawlik TM . Surgical Oncology.2018;27(2). CrossRef
- Heat Stress-Induced PI3K/mTORC2-Dependent AKT Signaling Is a Central Mediator of Hepatocellular Carcinoma Survival to Thermal Ablation Induced Heat Stress Thompson SM , Callstrom MR , Jondal DE , Butters KA , Knudsen BE , Anderson JL , Lien KR , et al . PloS One.2016;11(9). CrossRef
- Microsatellite Instability and Metastatic Colorectal Cancer – A Clinical Perspective Buchler T. Front Oncol.2022;:12. Available from: https://www.frontiersin.org/journals/oncology/articles/10.3389/fonc.2022.888181.
- Association of chemokine (CXC motif) receptor 4 expression with lymphovascular invasion and lymph node metastasis of invasive breast cancer Mamonto L, Nelwan BJ , Sungowati NK , Miskad UA , Cangara MH , Zainuddin AA . Breast Disease.2022;41(1). CrossRef
- Factors associated with Leptospira Serodiagnosis in febrile patients at public Health Centers in Makassar, Indonesia: a cross-sectional study Cahyaningtyas C, Muslich , Madjid B, Sultan , Hamid F, Hatta M. Pan African Medical Journal.2024;:49. CrossRef
- Comparison of interleukin 6 levels in patients with Hirschsprung-associated enterocolitis based on histopathological grade Husni Ma , Habar Tr , Mariana N, Kusuma Mi , Ahmadwirawan A, Faruk M. ResearchGate.2025. CrossRef
- Age-adjusted incidence rates of synchronous liver metastases for stage IV colorectal cancer compared by sex, race, and age group Ituarte PHG , Nelson R, O'Leary MP , Raoof M, Singh G. HPB: the official journal of the International Hepato Pancreato Biliary Association.2022;24(7). CrossRef
- IDDF2021-ABS-0191 Gender matters: sex disparities in colorectal cancer liver metastasis survival: a population-based study Wu Y, Xu W, Wang L. Gut.2021;70(Suppl 2). CrossRef
- Discordance of KRAS Mutational Status between Primary Tumors and Liver Metastases in Colorectal Cancer: Impact on Long-Term Survival Following Radical Resection Ardito F, Razionale F, Salvatore L, Cenci T, Vellone M, Basso M, Panettieri E, Calegari MA , Tortora G, Martini M, Giuliante F. Cancers.2021;13(9). CrossRef
- Novel molecular classification of colorectal cancer and correlation with survival Mangi FH , Shaikh TA , Soria D, Waryah AM , Ujjan ID , Qureshi JN , Syed BM . Saudi Journal of Biological Sciences.2022;29(5). CrossRef
- Risk and prognostic factors in patients with colon cancer with liver metastasis Gao J, Zhuang L, He C, Xu X, Zhu Z, Chen W. The Journal of International Medical Research.2023;51(9). CrossRef
- Biomarkers in colorectal liver metastases: Rising complexity and unknown clinical significance? Diener MK , Fichtner-Feigl S. Annals of Gastroenterological Surgery.2021;5(4). CrossRef
- Tumor location matters, next generation sequencing mutation profiling of left-sided, rectal, and right-sided colorectal tumors in 552 patients Ciepiela I, Szczepaniak M, Ciepiela P, Hińcza-Nowak K, Kopczyński J, Macek P, Kubicka K, Chrapek M, Tyka M, Góźdź S, Kowalik A. Scientific Reports.2024;14(1). CrossRef
- Impact of primary colorectal Cancer location on the KRAS status and its prognostic value Xie M, Li J, Cai Z, Li K, Hu B. BMC gastroenterology.2019;19(1). CrossRef
- The Clinical Significance of Microsatellite Instability in Patients with Right-sided Colorectal Cancer Han SA , Kim JH , Choi JH , Lee DH , Jung K, Kim SE , Moon W, Park MI , Park SJ . The Korean Journal of Gastroenterology = Taehan Sohwagi Hakhoe Chi.2019;73(3). CrossRef
- Clinicopathologic characteristics of left-sided colon cancers with high microsatellite instability Kim SK , Choi J, Kim HK , Park YN , Song SY , Kim H. Korean Journal of Pathology.2009;43(5). CrossRef
- Right Versus Left Colon Cancer Biology: Integrating the Consensus Molecular Subtypes Lee MS , Menter DG , Kopetz S. Journal of the National Comprehensive Cancer Network: JNCCN.2017;15(3). CrossRef
- Clinical, pathological, and adjuvant chemotherapy use differences among microsatellite unstable and microsatellite stable colon cancers Jafry BH , Buhaya MH , Milsap A, Jones AL , Goksu SY , Verma N, Brown TJ , Hughes A, Nair R, Sanford N, Su J, Huang E, Kazmi SMA . Journal of the National Cancer Center.2024;4(2). CrossRef
- A pan-cancer statistical study of microsatellite instability and Lynch syndrome–associated mismatch repair genes germline mutations. Cao H, Peisu S, Zhang W, Fang G, Li Y, Chen S, Chunze Z. Journal of Clinical Oncology.2023;41(16_suppl). CrossRef
- 141P Microsatellite instability and its relationship with systemic inflammation as prognostic factors in localised colorectal cancer Yeo J. H., Park J. H., Ahn H. K., Park I., Kim Y. S., Cho E. K., Shin D., Lee W.-S., Baek J. H., Sym S. J.. Annals of Oncology.2023;34. CrossRef
- Left-Sided microsatellite unstable colorectal cancers show less frequent methylation of hMLH1 and CpG island methylator phenotype than right-sided ones Tanaka J, Watanabe T, Kanazawa T, Tada T, Kazama Y, Tanaka T, Nagawa H. Journal of Surgical Oncology.2007;96(7). CrossRef
- Microsatellite Instability (MSI) as an Independent Predictor of Pathologic Complete Response (PCR) in Locally Advanced Rectal Cancer: A National Cancer Database (NCDB) Analysis Hasan S, Renz P, Wegner RE , Finley G, Raj M, Monga D, McCormick J, Kirichenko A. Annals of Surgery.2020;271(4). CrossRef
- Microsatellite instability: a review of what the oncologist should know Li K, Luo H, Huang L, Luo H, Zhu X. Cancer Cell International.2020;20. CrossRef
- KRAS mutations in colorectal cancer from Tunisia: relationships with clinicopathologic variables and data on TP53 mutations and microsatellite instability Aissi S, Buisine M, Zerimech F, Kourda N, Moussa A, Manai M, Porchet N. Molecular Biology Reports.2013;40(11). CrossRef
- KRAS Mutation in Gastric Cancer and Prognostication Associated with Microsatellite Instability Status Polom K, Das K, Marrelli D, Roviello G, Pascale V, Voglino C, Rho H, Tan P, Roviello F. Pathology oncology research: POR.2019;25(1). CrossRef
- 553O Prognostic value of KRAS and BRAF mutations in microsatellite stable (MSS) and unstable (MSI) stage III colon cancer: An ACCENT/IDEA pooled analysis of 7 trials Taieb J, Pederson L, Sinicrope FA , Lonardi S, Alberts S, et al T, et al . ResearchGate.2024. CrossRef
- Histopathological identification of colon cancer with microsatellite instability Alexander J., Watanabe T., Wu T. T., Rashid A., Li S., Hamilton S. R.. The American Journal of Pathology.2001;158(2). CrossRef
- Molecular genetic characteristics of colorectal cancer depending on the status of microsatellite instability Oganyan KA , Musaelyan AA , Kotikova MA , Lapin SV , Nazarov VD , Belyaev MA , et al . ResearchGate.2024. CrossRef
- Microsatellite Instability and KRAS Mutation in Stage IV Colorectal Cancer: Prevalence, Geographic Discrepancies, and Outcomes From the National Cancer Database Uhlig J, Cecchini M, Sheth A, Stein S, Lacy J, Kim HS . Journal of the National Comprehensive Cancer Network: JNCCN.2021;19(3). CrossRef
- BRAF-mutant microsatellite-stable rectal cancer with acquired KRAS mutation leading to drug resistance in liver metastasis Shigeyasu K, Yamamoto H, Takahashi T, Moriwake K, Kayano M, Takeda S, Matsumi Y, Umeda Y, Kondo Y, Teraishi F, Yasui K, Fuji T, Kagawa S, Fujiwara T. International Cancer Conference Journal.2024;13(3). CrossRef
- Practical Implications of KRAS Mutation Status and Sidedness of Primary Tumour in Patients with Colorectal Cancer and Synchronous Liver Metastases: A Subset Analysis of the CoSMIC Study Chan AKC , Siriwardena AK . Cancers.2022;14(19). CrossRef
- The Association of KRAS Mutation and Primary Tumor Location with Recurrence in Colorectal Liver Metastases Undergoing Radiofrequency Ablation Jiang B, Wang J, Yan K, Zhang Z, Wang S, Wu W, et al . 2023. CrossRef
- The interplay of KRAS mutational status with tumor laterality in non-metastatic colorectal cancer: An international, multi-institutional study in patients with known KRAS, BRAF, and MSI status Kamphues C, Kadowaki S, Amini N, Berg I, Wang J, Andreatos N, Sakamoto Y, et al . Journal of Surgical Oncology.2021;123(4). CrossRef
- Association of KRAS G12C Status with Age at Onset of Metastatic Colorectal Cancer Sunagua Aruquipa M, D'Alpino Peixoto R, Jacome A, Cesar F, Lorandi V, Dienstmann R. Current Issues in Molecular Biology.2024;46(2). CrossRef
- Codon-specific KRAS mutations predict overall survival benefit of trifluridine/tipiracil in metastatic colorectal cancer van de Haar J, Ma X, Ooft SN , van der Helm PW , Hoes LR , Mainardi S, et al . Journal of Clinical Oncology.2022 Jun 1;40(16_suppl):3593–3593. CrossRef
- Characterization of genomic alterations in Chinese colorectal cancer patients with liver metastases Wang H, Yan X, Wang L, Zhang M, Yang C, Wei-Liu n, Jin K, et al . Journal of Translational Medicine.2021;19(1). CrossRef
- Critical appraisal of surgical margins according to KRAS status in liver resection for colorectal liver metastases: Should surgical strategy be influenced by tumor biology? Rhaiem R, Duramé A, Primavesi F, Dorcaratto D, Syn N, Rodríguez ADLH , Dupré A, et al . Surgery.2024;176(1). CrossRef
- Abstract 519: Clinical impact of KRAS G12, G13, Q61, K117 and A146 mutations in patients with colorectal liver metastases van ’t Erve I, Wesdorp NJ , Medina JE , Ferreira L, Leal A, Huiskens J, et al . Cancer Res. 2022 Jun 15;82(12_Supplement):519–519. CrossRef
- Can the presence of KRAS mutations guide the type of liver resection during simultaneous resection of colorectal liver metastasis? Choi M, Han DH , Choi JS , Choi GH . Annals of Hepato-Biliary-Pancreatic Surgery.2022;26(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details