Production, Purification, and Characterization of L-Methioninase Produced by Escherichia coli Isolates: Potential Application in Cancer Treatment
Download
Abstract
Background: L-methioninase is one of the fascinating enzymes, and it has recently been the subject of intensive research due to its several potential medical uses, L-methionine plays a crucial role in the growth and proliferation of many cancer cells, particularly those seen in specific tumor types. L-methioninase’s ability to lower the body’s levels of L-methionine is the main reason for its strong anti-cancer effects.
Objective: To purify and characterize L-methioninase produced by Escherichia coli.
Methods: Twenty-one E. coli isolates were screened for L-methioninase production by semi-quantitative and quantitative methods, and then L-methioninase was purified using ammonium sulfate, ion exchange, and gel filtration chromatography.
Results: Out of all isolates, 16/21 (76.19%) were L-methioninase producers by semi-quantitative, while by quantitative screening, only six isolates out of these 21 isolates revealed specific activity ranged from 0.89 to 1.15 U/mg, and the maximum specific activity was for E. coli U8. The L-methioninase was purified using ammonium sulfate, ion exchange, and gel filtration chromatography. The best saturation ratio for L-methioninase precipitation was at 70% ammonium sulfate, considered a partial purification where L-methioninase specific activity was 2.52 U/mg. while L-methioninase-specific activity reached 4.11 U/mg when ion exchange chromatography was used with 3.2-fold purification and a yield of 46.2%. Finally, a pure L-methioninase-specific activity of 6.48 U/mg was gained using gel filtration with 5.1-fold purification and a yield of 42.9%. Determining molecular weight characterized the purified L-methioninase; the result showed that the purified L-methioninase had a molecular 50 kDa.
Conclusions: L-methioninase was purified from E. coli by many steps, including ammonium sulfate precipitation, DEAE-cellulose, and Sephedex G-150, in addition, SDS-PAGE electrophoresis supports the success of the purification process.
Introduction
In modern medicine, the rise of bacterial infections presents serious difficulties, especially in the context of the growing antibiotic resistance. Bacteria cause a significant number of hospital-acquired infections and mortality rates that are comparable to those of other serious health emergencies. This circumstance emphasizes the urgent need for creative ways to address this situation [1]. While Escherichia coli is frequently linked to pathogenicity, it is essential to consider that some strains can be used for beneficial purposes, such as purifying valuable substances like L-methioninase [2].
L-methioninase or methionine gamma-lyase enzyme (EC 4.4.1.11), belongs to the family of pyridoxal L–phosphate (PLP) dependent enzymes, which is familiar to catalyze alpha, gamma elimination of methionine to create alpha–ketobutyrate, methanethiol, and ammonia [3]. L-methioninase is a cytosolic enzyme inducing created via a methionine supplement to the culture medium [4].
L-methioninase’s ability to lower the body’s levels of L-methionine is the main reason for its strong anticancer effects. L-methionine plays a crucial role in the growth and proliferation of many cancer cells, particularly those seen in specific tumor types. L-methioninase can cause lethal effects in cancer cells by decreasing the availability of this vital amino acid while preserving normal cells that are less dependent on methionine [5]. L-methionine depletion has several downstream effects. First, it causes apoptosis; a decrease in methionine levels might cause cancer cells to initiate apoptotic pathways, resulting in programmed cell death. Second, it slows down or stops the growth of cancer cells by reducing the supply of a vital nutrient, which limits cell proliferation [5, 6].
Moreover, it modifies cellular metabolism. Cancer cells adapt their metabolic pathways to best use the available nutrients. However, L-methioninase interferes with this adaptation, resulting in metabolic stress and perhaps cell death [5, 6]. The enzyme’s potential as a treatment for several cancers, including the breast, prostate, and pancreas, has been investigated. Furthermore, the potential for large-scale manufacturing and therapeutic use of L-methioninase is highlighted by its potential to be produced from bacteria [7, 8].
Pyridoxal phosphate is the only cofactor employed by the L-methioninase enzyme. Pyridoxal phosphate lowers the energy required to transform amino acids. The apoenzyme stimulates the split of the substrate bond and yields the output [9]. L-methioninase is intracellularly present in bacteria. Gram-negative and Gram-positive bacteria have reportedly produced L-methioninase from fungal species as an extracellular and intracellular enzyme. The enzyme is not present in mammalian systems [10].
L-methioninase was isolated from different sources, including bacteria, fungi, protozoans, archaeons, and plants, except mammals [11]. This enzyme has been demonstrated to be present in various bacteria, such as Serratia marcescens, Bacillus subtilis, E. coli, Klebsiella oxytoca, Klebsiella pneumonia, Citrobacter intermedius, Citrobacter freundii, Aeromonas sp. [12], Brevibacterium linens [13], Pseudomonas spp. [14], Clostridium sporogenes [15], and the plant Arabidopsis thaliana [16]. Fungus like Aspergillus flavipes, Penicillium notatum, and Fusarium solani are also in the protozoan Entamoeba histolytica [17] and Trichomonas vaginalis [18]. This research aimed to detect the L-methioninase activity in E. coli, in addition to the purification of L-methioninase and detection of its molecular weight.
Materials and Methods
Collection and Identification of Bacterial Isolates
Twenty-one clinical isolates of E. coli were collected from different hospitals in Baghdad city. These bacterial isolates were obtained from different clinical specimens, including urine, wound, sputum, blood, and pus. The specimens underwent immediate subculture on MacConkey agar and incubated at 37 °C for 18-24 hours. All bacterial isolates were identified depending on their microscopic characteristics, cultural characteristics [19], and biochemical tests [20] and then approved using the VITEK 2 compact system.
Detection Methods for L-methioninase Production
L-methioninase Production detection was conducted using semi-quantitative and quantitative methods, as described by Al-Zahrani and Bukhari [21] and Hanniffy et al. [22], respectively. All E. coli isolates were inoculated to a modified M9 agar medium in the semi-quantitative method. Results were examined after post-incubation of plates at 37°C for 48 hours. The appearance of a yellow zone around the colonies considered L-methioninase production, and then the diameters of clear zones around the colonies were measured [21]. Also, in the quantitative method, the chosen E. coli isolates were inoculated to a modified M9 broth medium and incubated at 37°C for 24 hours. 10 mL of activated bacteria was inoculated in 100 mL of modified M9 broth medium and incubated for 48 hours at 37ºC. After centrifugation at 4000 rpm for 30 minutes, the supernatant was discarded, and the pellet was washed twice with 20 mM potassium phosphate buffer at pH 7.2. The cells were resuspended in potassium phosphate (20mM, at pH 7.2)-EDTA-PMSF-2- mercaptoethanol buffer. The enzyme was extracted from E. coli cells by rupturing with glass beads, followed by the enzyme’s assessment activity [22].
L-methioninase Assay and Protein Quantification
The enzyme activity was tested by a partially modified 3-methyl-2-benzothiazolinone (MBTH) stopped spectrophotometric rate determination method [4]. For conducting this method, 0.02 mL of L-methioninase enzyme was added to a tube containing 2 mL of potassium phosphate buffer (100mM at pH 7) containing 25 mM L- methionine and 0.01 mM pyridoxal 5-phosphate which equilibrate to 37 ºC, mixed and incubated at 37 ºC for 10 minutes. The reaction was stopped by adding 0.25 mL of 50% TCA. One mL of the test reaction was transferred to another new tube containing 2 mL of sodium acetate buffer (1M at pH 5), then 0.8 mL of (MBTH) was added with mixing and incubated at 50 ºC for 30 minutes, followed by 30 minutes’ post-incubation at 25 ºC. The amount of produced α-ketobutyrate was measured spectrophotometrically based on the increase in absorbance at 320 nm from the blank. Bradford protein assay method was used for protein quantification [23] with bovine serum albumin as standard. The L-methioninase-specific activity was expressed as the enzyme’s activity in units per milligram of protein [3].
L-methioninase Purification
The L-methioninase purification was performed as suggested by Yaguchi et al. [24]. The crude extract was exposed to fractionate to 20-90% ammonium sulfate. At 4°C, all samples were left overnight, and then by centrifugation at 10000 rpm for 15 min, the precipitates were collected and dissolved in potassium phosphate buffer pH 7, then dialyzed overnight with the same buffer. The sample was loaded onto the DEAE-cellulose column equilibrated with potassium phosphate buffer, pH 7, and eluted with a 0.1-1 M NaCl gradient made in the same buffer.
Fractions (5 ml) were gathered and examined L-methioninase enzyme activity, then applied to Sephadex G-150 column (2 x 40 cm) that was pre-equilibrated and washed with potassium phosphate buffer and the elution was done by the same buffer. Protein concentration at 280 nm, L-methioninase activity, and specific activity was determined, and the active fractions were pooled for further experiments.
Determination of the Molecular Weight
The aid of standard proteins could estimate the molecular weight of the enzyme. The molecular weight of L-methioninase was determined by using SDS-PAGE [25].
Results
Collection and identification of bacterial isolates
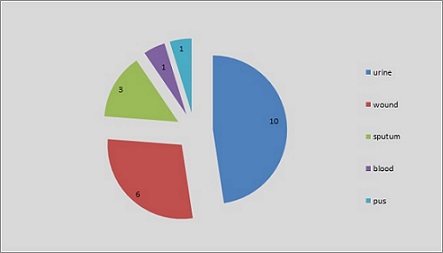
The results showed that most collected clinical E. coli isolates were obtained from urine infections with ten isolates, wound infections with six isolates, followed by sputum infections with three isolates in comparison with blood and pus infections that were with less number with one isolate as displayed in Figure 1.
Figure 1. Number of Collected E. coli Isolates Depends on the Source of Isolation.
L-methioninase Production Detection
In this study, the E. coli isolates were tested for L-methioninase production using semi-quantitative and quantitative analysis. A modified M9 agar medium was used for L-methioninase production in the semi-quantitative method. Depending on the diameter of the yellow zones, only 16 isolates had the ability to produce L-methioninase. These E. coli isolates ranged in diameter zone from 11 to 22 mm Figure (2A).
Figure 2. (A) Diameter Zone (mm) for E. coli Isolates (B) E. coli U8 Producer Isolate on Modified M9 Agar Medium. .
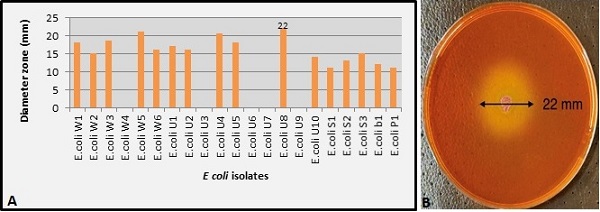
According to the results, E. coli U8 was the most potent producer of L-methioninase with the highest diameter (22 mm) and intensity yellow zone, as shown in Figure 2B.
The basic pH change is shown by phenol red, which turns yellow in acidic and red in alkaline conditions. Similarly, this technique is used to study the dissimilation of L-methioninase for L-methionine. Therefore, on the modified M9 medium, the isolates showing the yellow zone were selected for primary detection.
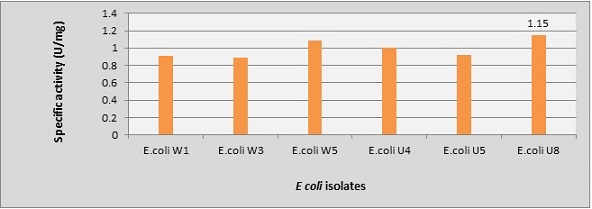
In the quantitative method, we chose only six of these 16 isolates that gave a larger hydrolysis yellow zone. These six isolates revealed different levels of L-methioninase production with specific activities ranging from 0.89 to 1.15 U/mg, with maximum L-methioninase activity equal to 1.74 U/ml and 1.15 U/mg of specific activity by E. coli U8 (Figure 3).
Figure 3. Specific Activities for Selected E. coli Isolates Were Performed Using a Quantitative Method of Modified M9 Broth..
The amount of α -ketobutyrate produced was determined spectrophotometrically using MBTH (3-Methyl-2-Benzothiazoline Hydrazone). MBTH reacts with the α-ketobutyrate end product to form a colored product [6].
L-methioninase purification
L-methioninase is an intracellular enzyme in the culture broth of E. coli. The three steps of L-methioninase purification are summarized in Table 1.
| Purification step | Volume (mL) | Enzyme activity (U/mL) | Protein concentration (mg/mL) | Specific activity (U/mg) | Total activity (U) | Purification (folds) | Yield (%) |
| Crude extract | 40 | 2.99 | 2.38 | 1.26 | 119.6 | 1 | 100 |
| Ammonium sulphate precipitation (70%) | 20 | 3.68 | 1.46 | 2.52 | 73.6 | 2 | 61.5 |
| DEAE-cellulose | 14 | 3.95 | 0.96 | 4.11 | 55.3 | 3.2 | 46.2 |
| Sephadex | 12 | 4.28 | 0.66 | 6.48 | 51.36 | 5.1 | 42.9 |
| G-150 |
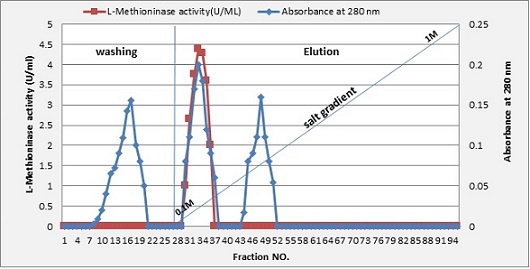
The first purification step was carried out by protein precipitation from the cell-free supernatant using ammonium sulphate at a 70% saturation ratio with L-methioninase- specific activity of 2.52 U/mg. The solution gathered from ammonium sulphate precipitation was loaded on DEAE-cellulose and eluted using a sodium chloride gradient. In this step, L-methioninase was purified 3.2 fold, yielding about 46.2%. The L-methioninase activity gained from this step was 3.95 U/mg, as shown in Figure 4.
Figure 4. Ionic Exchange Chromatography for L-methioninase by DEAE Cellulose Column (3 x15) cm. The column was calibrated with potassium phosphate buffer pH7, 5 ml fraction, and 60 ml/hrs flow rate.
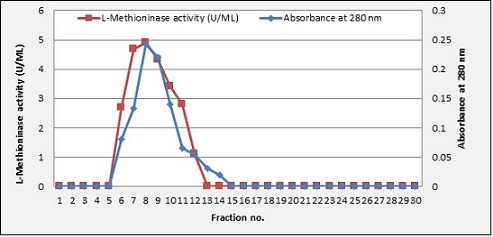
The procedure of purification was completed by the Sephadex G-150 column. The results showed that L-methioninase was purified with 5.1 fold purification and a yield of 42.9%, obtaining a final specific activity of 6.48 U/mg, as shown in Figure 5.
Figure 5. Gel Filtration Chromatography by Using Sephadex G-150 Column (2x40) cm for Purified L-methioninase. The column was calibrated with potassium phosphate buffer pH 7; 3 ml/fraction and flow rate 30 ml/hrs.
L-methioninase Molecular Weight
Electrophoresis using polyacrylamide gel was used to measure the degree of L-methioninase purity. After the gel filtration step, the protein profile for L-methioninase gave one band. The purified enzyme was electrophoresed under denaturing conditions using SDS-PAGE and stained with coomassie blue dye, confirming that it was purified to homogeneity. The L-methioninase molecular weight was 50 kDa when the marker proteins were compared, as shown in Figures 6 and 7.
Figure 6. SDS Polyacrylamide gel Electrophoresis of Purified L Methioninase Production from E. coli U8 (1): Proteins Marker (2) and (3): Gel filtration Protein band.
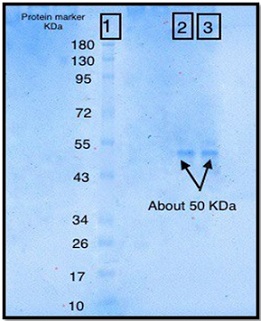
Figure 7. Determination of Molecular Weight of L-methioninase. Log (M.W.): logarithm of molecular weight, Rm= relative mobility, Da: Dalton.
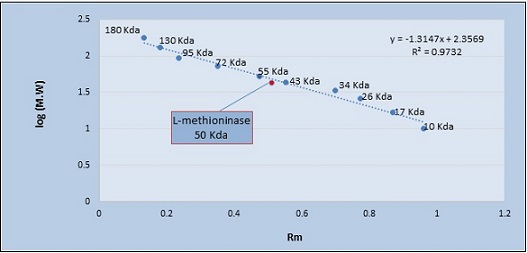
Over a molecular weight range determined by gel pore size, a linear relationship between log M.W. and mobility is obtained. Thus, the gel may be normalized using proteins of known molecular weight and then utilized to estimate the molecular weights of the unknowns.
Discussion
Despite advancements in cancer treatment, this disease remains a leading cause of mortality worldwide. Reducing the high mortality rate demands innovative and effective medicines. Current cancer therapies often have significant limitations, including adverse side effects and a chance of the development of resistance, which can reduce treatment outcomes and patients’ quality of life. Therefore, there is a need for novel anti-cancer therapeutic agents [26]. Due to the anti-cancer properties of L-methioninase this amino acid the potential of being a proper cancer medicine of this amino acid must not be overlooked.
In this study 16 E. coli isolates with potential of producing L-methioninase isolated from different medical specimens. According to the results, E. coli U8 have the highest potential in producing of L-methioninase. The production of α-keto butyric acid causes a pH decrease, which causes the color to change to yellow. The result agrees with [27-29].
The highest L-methioninase activity in our study was equal to 1.74 U/ml that is belongs to E. coli U8. Bopaiah et al. [10] investigated that L-methioninase produced from Bacillus haynesii, which was isolated from different locations, gave a higher yield of L-methioninase and it was chosen based on hydrolytic zone formation around the colonies and the highest activity level of L-methioninase was recorded with 7.38 U/ml and specific activity of 9.22 U/mg. Significant differences in yield and specific activity were found when comparing the production of L-methioninase between our work and that of Bopaiah et al. [10] According to these results, B. haynesii may naturally be more effective than E. coli in producing L-methioninase in comparable circumstances.
Moreover, enzyme expression levels and metabolic characteristics unique to a species may be the cause of the observed variations in yield and specific activity. It’s possible that B. haynesii has a genetic and metabolic composition that enables it to generate more L-methioninase, perhaps as a result of more efficient enzyme synthesis pathways. Additionally, the overall yield may be affected by extracellular or intracellular production location differences between B. haynesii and E. coli.
In a similar study in which L-methioninase was purified from isolates of Bacillus haynesii JuB₂, the highest specific activity mentioned was 102.15 U/mg with purification folds of about 11.07 [10]. Another study [30] reported that L-methioninase was purified from a Streptomyces sp. by spandex G-100, resulting in a specific activity of 260.5 U/mg with a purification fold of about 3.15. L-methioninase from Aspergillus fumigatus was purified using a DEAE-cellulose exchanger, and its specific activity was 23.74 U/mg and had a fold of purification, giving rise to 1.76 with 81.2% of recovery yield [31]. Also, El-Shora et al. [32] mentioned that the purification fold reached 6.5, 22.6 U/mg specific activity, and 55.6% recovery after ion exchange chromatography for the MGL enzyme purified from Aspergillus flavipes. Compared with the mentioned studies, our results show that the specific activity and yield of L-methioninase are lower than those from other microbiological sources. For instance, Streptomyces sp. showed even more significant activity, with 260.5 U/mg after purification with a 3.15-fold purification fold [30], while B. haynesii isolate JuB₂ obtained a specific activity of 102.15 U/mg with an 11.07-fold purification in Bopaiah et al.’s investigation [10]. These variations imply that some organisms, like Streptomyces sp. and B. haynesii, have enzyme isoforms with naturally higher activity or naturally better efficiency for generating L-methioninase. Furthermore, the higher specific activities of L-methioninase from A. fumigatus [31] imply that fungal sources of the enzyme might have unique structural or biochemical characteristics that boost their activity compared to bacterial L-methioninase. These differences could be caused by differences in active site accessibility, cofactor binding efficiency, or enzyme folding. Furthermore, there could be several reasons for the comparatively decreased specific activity of L-methioninase that we found in our investigation. Compared to Bacillus and Streptomyces, E. coli may express L-methioninase in less catalytically active forms.
Despite effectiveness, the purification process may also contribute to these differences. Separate chromatography methods, like Sephadex G-150, might not produce the same degree of purity and activity as those employed in the other studies, which used additional or different chromatography steps. Furthermore, the intrinsic stability of L-methioninase from various species can influence the final yield and activity after purification. For example, enzymes from particular strains of Bacillus, Streptomyces, and Aspergillus may be more robust during purification, maintaining higher activity.
Abdelraof et al. [30] indicated that purified Streptomyces DMMMH60 l-methioninase showed one SDS-PAGE band representing 55 kDa subunits. Conversely, Trichoderma harzianum produces L-methioninase, which has an apparent molecular weight of 48 kDa and is represented by a single band [33]. While the produced L-methioninase. In our study shows 50kDa. Species-specific changes in the gene sequences encoding L-methioninase may be the reason behind the observed differences in molecular weight among various species. These different variations may result in minor differences in the structure of the enzyme. Furthermore, post-translational modifications fluctuate among bacterial and fungal species as well as between prokaryotic and eukaryotic organisms, which affects the enzyme’s ultimate molecular weight. A conserved structural framework for L-methioninase across species is suggested by the 50 kDa molecular weight found in our E. coli -derived enzyme, with minor differences probably caused by these genetic and metabolic variables. Additionally, our study’s L-methioninase single-band profile on SDS-PAGE indicates suitable purification. It implies that the enzyme occurs as a monomer, resembling the forms reported for Streptomyces and Trichoderma. This conserved monomeric structure highlights the structural and functional similarities of L-methioninase across several taxa, despite slight size variations. Future research, including in-depth structural analysis, may shed more light on how these subtle differences in molecular weight affect the stability, activity, and function of enzymes.
In conclusion, L-methionine plays a crucial role in the growth and proliferation of many cancer cells, particularly those seen in specific tumor types. L-methioninase can cause lethal effects in cancer cells by decreasing the availability of this vital amino acid while preserving normal cells that are less dependent on methionine. In the current study, the production and multi-step purification of L-methioninase from E. coli is successfully demonstrated, yielding a 42.9% yield and a 5.1-fold purification. The enzyme’s single-band profile at 50 kDa validates its purity, making this method a dependable way to obtain L-methioninase for additional biochemical and therapeutic applications, especially in cancer treatment.
Acknowledgments
None.
Conflict of Interests
The authors declared no conflict of interest.
Funding source
The authors did not receive any source of funds.
Data sharing statement
Supplementary data can be shared with the corresponding author upon reasonable request.
References
- A New Insight into Phage Combination Therapeutic Approaches Against Drug-Resistant Mixed Bacterial Infections Rahimian M, Jafari-Gharabaghlou D, Mohammadi E, Zarghami N. PHAGE (New Rochelle, N.Y.).2024;5(4). CrossRef
- L-methioninase enzyme production by E. coli WSM2 using some organic by product residues Baghdadi AM , Danial EN . Egyptian Journal of Chemistry.2022. CrossRef
- L-methioninase production by Aspergillus flavipes under solid-state fermentation El-Sayed ASA . Journal of Basic Microbiology.2009;49(4). CrossRef
- Assay method for antitumor L-methionine gamma-lyase: comprehensive kinetic analysis of the complex reaction with L-methionine Takakura T, Mitsushima K, Yagi S, Inagaki K, Tanaka H, Esaki N, Soda K, Takimoto A. Analytical Biochemistry.2004;327(2). CrossRef
- A review on L-methioninase in cancer therapy: Precision targeting, advancements and diverse applications for a promising future Javia BM , Gadhvi MS , Vyas SJ , Ghelani A, Wirajana N, Dudhagara DR . International Journal of Biological Macromolecules.2024;265(Pt 2). CrossRef
- An Extensive Review on L - Methioninase and Its Potential Applications Suganya K, et al . Biocatalysis and Agricultural Biotechnology.2017;:104-115. CrossRef
- Effect of physicochemical parameters on the L-methioninase activity of Methylobacterium sp. and its in vitro anticancer activity in combination with tamoxifen citrate Dayanand K, Nadumane V. Future Journal of Pharmaceutical Sciences.2023;9. CrossRef
- Bioprospecting of a Thermostable L-Methioninase from Alcaligenes aquatilis BJ-1 in Agro-Industrial Waste Javia B, Gadhvi M, Vyas S, Dudhagara P, Shyu D, Chen Y, Dudhagara D. Microbiology Research.2023. CrossRef
- Benchmark Reaction Rates, the Stability of Biological Molecules in Water, and the Evolution of Catalytic Power in Enzymes Wolfenden R. Annual review of biochemistry.2011;80. CrossRef
- Purification, characterization, and antiproliferative activity of L-methioninase from a new isolate of Bacillus haynesii JUB2 Kotramada B, Nanda K, Kohini B, Lucky D, Venkatesh R, Suresh A, Nadumane V. Journal of Applied Pharmaceutical Science.2020;10. CrossRef
- Structure of the antitumour enzyme L-methionine gamma-lyase from Pseudomonas putida at 1.8 A resolution Kudou D, Misaki S, Yamashita M, Tamura T, Takakura T, Yoshioka T, Yagi S, et al . Journal of Biochemistry.2007;141(4). CrossRef
- Microbial Enzymes and Metabolites for Health and Well-Being Sirohi, Ranjna , Amit Kumar Rai , Luciana Porto de Souza Vandenberghe , Binod Parameswaran, eds . Boca Raton: CRC Press.2023. CrossRef
- “Cytotoxic Effect of L-Methioninase from Brevibacterium Linens BL2 in Combination with Etoposide against Glioblastoma Cells.” İpek, Semih Latif, , Meryem Damla Özdemir , Dilek Göktürk . Applied Sciences. Multidisciplinary Digital Publishing Institute.2023;13(16):9382. CrossRef
- Biochemical, molecular and anti-tumor characterization of L-methionine gamma lyase produced by local Pseudomonas sp. in Egypt Abou Zeid AA , Mohamed AH , El-Sayed ASA , El-Shawadfy AM . Saudi Journal of Biological Sciences.2023;30(7). CrossRef
- Methionine gamma lyase from Clostridium sporogenes increases the anticancer effect of doxorubicin in A549 cells and human cancer xenografts Pokrovsky V. S., Yu Anisimova N., Zh Davydov D., Bazhenov S. V., Bulushova N. V., Zavilgelsky G. B., Kotova V. Y., Manukhov I. V.. Investigational New Drugs.2019;37(2). CrossRef
- “Purification and Biochemical Characterization of L-Methioninase from Fenugreek.” Reda Alaa M, Neven Ahmad Sala , Magdy M, Yousef , Hamed M, El-Shora . Egyptian Journal of Chemistry.2023;66(11):25-31. National Information and Documentation Centre (NIDOC), Academy of Scientific.
- Genetic, metabolomic and transcriptomic analyses of the de novo L-cysteine biosynthetic pathway in the enteric protozoan parasite Entamoeba histolytica Jeelani G, Sato D, Soga T, Nozaki T. Scientific Reports.2017;7(1). CrossRef
- Microbial L-methioninase: production, molecular characterization, and therapeutic applications El-Sayed AS . Applied Microbiology and Biotechnology.2010;86(2). CrossRef
- Characteristics of diarrheagenic Escherichia coli pathotypes among children under the age of 10 years with acute diarrhea Shahbazi G, Rezaee MA , Nikkhahi F, Ebrahimzadeh S, Hemmati F, Namarvar BB , Gholizadeh P. Gene Reports.2021;25. CrossRef
- Extraction and purification of lectin from rice grains and using it as a novel prebiotic and inhibitor toward some gastrointestinal organisms Muslim S, Hassan Muslem W, Fayyad R, Al A, Edbeib M. Bionatura.2023;8. CrossRef
- “Genetics Identification and Detection of L-Methioninase Gene in a Pseudomonas Isolate.” Al-Zahrani, S. H. M , K. A. Bukhari . Pharmacophore.2019;10(6):93-98.
- Heterologous Production of Methionine-γ-Lyase from Brevibacterium linens in Lactococcus lactis and Formation of Volatile Sulfur Compounds Hanniffy SB , Philo M, Peláez C, Gasson MJ , Requena T, Martínez-Cuesta M. C.. Applied and Environmental Microbiology.2009;75(8). CrossRef
- Broad-Spectrum Biofilm and Adhesion Inhibition by Levanase Purified from Enterobacter aerogenes Enterobacter aerogenes Muslim S. Al-Mustansiriyah Journal of Science.2015;26. CrossRef
- Preparation of κ-Casein by Gel Filtration Yaguchi M, Davies D. T., Kim Y. K.. Journal of Dairy Science.1968;51(4). CrossRef
- Production and Partial Purification of Tannase from Serratia Marcescens Isolated from Different Sources Muslim S. Al-Mustansiriyah Journal of Science.2018;28. CrossRef
- A View on Drug Development for Cancer Prevention Reynolds AR , Moschetta M, Yohannes AR , Walcott F, Ashford M, Szucs Z, Sarbajna T, er al . Cancer Discovery.2023;13(5). CrossRef
- Comparative study of a new alkaline L-methioninase production by Aspergillus ustus AUMC 10151 in submerged and solid-state fermentation Abu-Tahon M, Isaac G. Brazilian Archives of Biology and Technology.2016;59. CrossRef
- l-Methioninase from some Streptomyces isolates I: Isolation, identification of best producers and some properties of the crude enzyme produced Selim M. H., Elshikh H. H., El-Hadedy D. E., Saad M. M., Eliwa E., Abdelraof M.. Journal, Genetic Engineering & Biotechnology.2015;13(2). CrossRef
- Isolation and characterization of Trichoderma harzianum L-methioninase with promising a powerful anticancer Ashkan MF , Younis SA , Elazab NT . Saudi Journal of Biological Sciences.2023;30(12). CrossRef
- Statistically optimized production of extracellular L-methionine γ-lyase by Streptomyces Sp. DMMMH60 and evaluation of purified enzyme in sub-culturing cell lines Abdelraof M., Helmy S, Abo Elsoud M, Ali M. Biocatalysis and Agricultural Biotechnology.2019;18. CrossRef
- Purification, Characterization and anticancer activity of L-methionine γ-lyase from thermo-tolerant Aspergillus fumigatus Hendy MH , Hashem AH , Suleiman WB , Sultan MH , Abdelraof M. Microbial Cell Factories.2023;22(1). CrossRef
- Production, Optimization And Purification Of L-Methioninase From Aspergillus Flavipes AUMC 1201,” January El-Shora , Hamed , Alaa Abu-Zied , Omyma Awadalla , Sally Metwally , Nessma Elzawawy . 2021;:312-316.
- Process Modeling and Optimization of High Yielding L-Methioninase from a Newly Isolated Trichoderma Harzianum Using Response Surface Methodology and Artificial Neural Network Coupled Genetic Algorithm Salim Nish , A Santhiagu , K Joji . Biocatalysis and Agricultural Biotechnology 17 (January).2019;:299-308. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details