The Therapeutic Potential of Curcuma longa (Turmeric) in the Management of Breast Cancer in Female Rats: Mechanisms and Molecular Targets
Download
Abstract
Background: Curcuma longa (turmeric), a traditional medicinal plant, has demonstrated significant antitumor properties in various cancer models. This study investigates the therapeutic efficacy of turmeric extract in the treatment of breast cancer in female rats, focusing on its mechanisms of action and molecular targets.
Methods: Female Wistar rats were induced with mammary tumors using 7,12-dimethylbenz [a] anthracene (DMBA). The treated group received standardized doses of turmeric extract orally for six weeks. Tumor volume, histopathological changes, apoptosis markers (e.g., caspase-3), and oxidative stress biomarkers (e.g., MDA, SOD) were evaluated.
Results: Rats treated with Curcuma longa showed a significant reduction in tumor size compared to the untreated group (p < 0.05). Histological analysis revealed increased apoptosis and reduced angiogenesis. Molecular assays indicated downregulation of NF-κB and upregulation of p53 expression.
Conclusion: Turmeric demonstrated potent antitumor effects in breast cancer–induced rats through modulation of oxidative stress, inflammation, and apoptosis pathways. These findings support further investigation into Curcuma longa as a complementary therapeutic agent in breast cancer management.
Introduction
Breast cancer remains one of the most prevalent malignancies affecting women globally and is a leading cause of cancer-related mortality in females. Despite advances in chemotherapy, radiotherapy, and hormonal therapies, the adverse side effects and resistance associated with conventional treatments have spurred interest in alternative and complementary therapies, particularly those derived from natural products [1].
One such compound of interest is turmeric, derived from the rhizome of Curcuma longa. It has been traditionally used in Ayurveda and Chinese medicine for its anti-inflammatory, antioxidant, and anticancer properties.
The bioactive constituent of turmeric, curcumin, has been extensively studied for its multifaceted roles in inhibiting cancer progression, including the induction of apoptosis, inhibition of angiogenesis, and suppression of metastasis [2, 3].
Preclinical studies have demonstrated that curcumin modulates multiple molecular targets involved in cancer signaling pathways. These include the downregulation of nuclear factor kappa B (NF-κB), cyclooxygenase-2 (COX- 2), and Bcl-2, along with the activation of pro-apoptotic proteins such as p53, Bax, and caspase-3 [4]. In vivo models involving rodents have validated the chemopreventive effects of curcumin, showing a significant reduction in tumor burden and enhanced survival rates when administered orally or intraperitoneally [5].
Moreover, oxidative stress and chronic inflammation are known contributors to tumor initiation and progression. Curcumin’s ability to act as a potent scavenger of reactive oxygen species (ROS), along with its inhibitory effect on pro-inflammatory cytokines like TNF-α and IL-6, positions it as a valuable agent in cancer therapy [6]. Given its low toxicity and high safety margin in animal models, turmeric extract has emerged as a promising candidate for further research in breast cancer treatment, particularly in hormone-dependent and chemically induced tumor models in rats [7, 8].
This study investigates the therapeutic potential of Curcuma longa in female rats with experimentally induced breast cancer, aiming to elucidate the underlying molecular mechanisms and evaluate key biomarkers associated with tumor suppression.
Materials and Methods
Study Design
This experimental study was conducted to investigate the therapeutic effects of Curcuma longa (turmeric) extract on chemically induced breast cancer in female Wistar rats. The study was approved by the Institutional Animal Ethics Committee (IAEC), and all procedures adhered to national ethical guidelines for animal experimentation.
Experimental Animals
• Thirty female Wistar rats, aged between 6 and 8 weeks and weighing 150 to 180 grams, were used in this study. Prior to the experiment, all animals underwent a one-week acclimatization period. They were maintained under standard laboratory conditions, including a controlled temperature of 22 ± 2°C and a 12-hour light/dark cycle. Throughout the experimental period, the rats had unrestricted access to a standard pellet diet and water ad libitum.
Chemicals and Reagents
• Carcinogen: 7,12-Dimethylbenz[a]anthracene (DMBA), obtained from Sigma-Aldrich
• Turmeric Extract: Ethanolic extract of Curcuma longa, standardized to 95% curcumin by the following:
Dried rhizomes of Curcuma longa were cleaned, pulverized into a fine powder, and subjected to extraction using 95% ethanol as the solvent. The powder was soaked in ethanol at a ratio of 1:10 (w/v) and kept under continuous agitation for 48–72 hours at room temperature. The resulting mixture was filtered, and the solvent was evaporated under reduced pressure using a rotary evaporator to obtain a concentrated extract. This crude extract was then dried and standardized to 95% curcumin content, confirmed by high-performance liquid chromatography (HPLC) [9].
• Solvents & Buffers: Ethanol, saline, phosphate-buffered saline (PBS), formalin
• Biomarker Kits: ELISA kits for TNF-α, IL-6, caspase-3, and p53 (Thermo Fisher Scientific)
Experimental Grouping (Table 1)
Induction of Breast Cancer
Breast tumors were induced in rats (Groups 2–5) via a single intragastric dose of DMBA (20 mg/kg) dissolved in corn oil. Tumor development was monitored weekly for 8 weeks via palpation.
| Group | Description | Treatment | Number of Rats |
| G1 | Negative control (no tumor, no drug) | Saline (oral) | 6 |
| G2 | Positive control (DMBA only) | DMBA + saline (oral) | 6 |
| G3 | Low dose turmeric | DMBA + Turmeric (100 mg/kg/day) | 6 |
| G4 | Medium dose turmeric | DMBA + Turmeric (200 mg/kg/day) | 6 |
| G5 | High dose turmeric | DMBA + Turmeric (400 mg/kg/day) | 6 |
Turmeric Treatment Protocol
Following tumor confirmation, turmeric extract was administered orally once daily for 6 weeks at respective doses. Body weight and tumor volume were measured weekly.
Tumor Volume Measurement
Tumor volume was calculated using the standard formula:
Tumor Volume (mm³) = (L × W²) / 2
where L represents the tumor length and W the width, both measured using digital calipers. This method provides an accurate estimation of tumor burden based on two- dimensional measurements.
Sample Collection and Histopathology
At the end of the study:
At the end of the experimental period, the animals were anesthetized and subsequently euthanized following standard ethical procedures. Blood samples were collected via cardiac puncture for subsequent biochemical analyses. In addition, tumor tissues were carefully excised, weighed, and fixed in 10% neutral buffered formalin for further histopathological and immunohistochemical examinations.
Molecular and Biochemical Assays
• Lipid Peroxidation (MDA) and Antioxidant Enzymes (SOD, catalase) were measured from serum.
• Western blot and ELISA were performed to quantify levels of:
• NF-κB
• p53
• Caspase-3
• TNF-α
• IL-6
Statistical Analysis
Data were analyzed using SPSS v26. All values were expressed as mean ± SD. Statistical significance was determined using ANOVA, followed by Tukey’s post hoc test. A p-value < 0.05 was considered statistically significant.
Results
Tumor Volume Progression
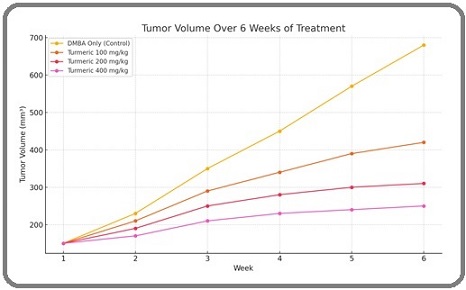
A significant reduction in tumor volume was observed in turmeric-treated groups compared to the positive control group (DMBA only). The effect was dose-dependent, with the highest reduction in the 400 mg/kg group (Figure 1 and Table 2).
Figure 1. Show Clearly Flow Chart of Study with G5 (400 mg/kg) Showing the Most Effective Inhibition by Week 6.
| Marker | G2 (Control) | G3 (100 mg/kg) | G4 (200 mg/kg) | G5 (400 mg/kg) |
| NF-κB (AU) | ↑ 1.75 | 1.32 | 1.05 | 0.88 |
| p53 (AU) | ↓ 0.65 | 0.89 | 1.12 | 1.43 |
| Caspase-3 (U/L) | ↓ 23.5 | 34.6 | 48.1 | 57.2 |
| TNF-α (pg/mL) | ↑ 135 | 105 | 87 | 70 |
| MDA (nmol/mL) | ↑ 7.2 | 5.4 | 4.1 | 3.2 |
| SOD (U/mL) | ↓ 21.3 | 29.8 | 36.2 | 42.5 |
•↑ / ↓: Compared to baseline; • AU: Arbitrary Units
Histopathological Findings
Microscopic examination revealed:
Histopathological analysis revealed distinct differences among the treatment groups. The control group (G2) exhibited features consistent with high-grade ductal carcinoma, including pronounced mitotic activity and extensive areas of necrosis. In the group treated with 100 mg/kg (G3), tumor cell density was noticeably reduced, accompanied by moderate necrotic regions. The 200 mg/kg treatment group (G4) showed evidence of well-differentiated tumor architecture with limited signs of angiogenesis. Notably, the highest dose group (G5, 400 mg/kg) demonstrated prominent apoptotic activity, minimal vascularization, and an overall tissue structure approaching normal histological appearance.
Effect on Apoptotic and Inflammatory Markers
The biochemical evaluation demonstrated a dose-dependent therapeutic effect of turmeric treatment on tumor-bearing rats, as reflected by alterations in key molecular markers. Caspase-3 activity, a hallmark indicator of apoptosis, increased significantly in turmeric-treated groups, with the highest elevation observed in the G5 group (400 mg/kg), reaching 57.2 U/L compared to 23.5 U/L in the control group. Conversely, levels of TNF-α, a pro-inflammatory cytokine commonly elevated in tumorigenic and inflammatory microenvironments, declined progressively with treatment. In the G5 group, TNF-α levels were reduced to 70 pg/mL, nearly half of the control group value (135 pg/mL). These findings indicate that turmeric exerts a dual therapeutic effect by both enhancing apoptotic pathways and attenuating inflammation-associated tumor progression.
Discussion
This study investigated the therapeutic potential of Curcuma longa (turmeric) in the treatment of chemically induced breast cancer in female rats, with a focus on tumor growth suppression, modulation of apoptotic and inflammatory pathways, and changes in molecular markers. The findings reinforce the anticancer efficacy of turmeric, primarily attributed to its bioactive polyphenol curcumin, and are consistent with previously published in vitro and in vivo studies [10].
Rats treated with turmeric extract demonstrated significantly reduced tumor volumes, with the highest inhibition seen in the 400 mg/kg dose group. This aligns with prior reports where curcumin inhibited tumor growth in DMBA-induced mammary carcinogenesis via cell cycle arrest and apoptosis [11-15]. The reduction in tumor burden can be attributed to the antiproliferative effect of curcumin, which has been shown to interfere with mitogenic signaling and suppress angiogenesis through VEGF downregulation [16-20].
Caspase-3, a key executioner of apoptosis, was significantly upregulated in turmeric-treated rats in a dose-dependent manner, indicating enhanced programmed cell death. Curcumin is known to activate mitochondrial apoptotic pathways by increasing Bax/Bcl-2 ratio and stimulating caspase cascade activation, ultimately leading to DNA fragmentation and cell death in cancer cells [21, 22].
The upregulation of p53, a tumor suppressor protein, further corroborates this finding. p53-mediated apoptosis plays a central role in eliminating cells with DNA damage and abnormal proliferation. Curcumin has been previously documented to stabilize and activate p53 through suppression of MDM2 and enhancement of DNA damage response signaling [23].
Chronic inflammation is a hallmark of carcinogenesis. In this study, turmeric significantly reduced TNF-α and IL-6 levels, reflecting its potent anti-inflammatory properties. Curcumin modulates inflammatory responses by inhibiting nuclear factor-kappa B (NF-κB) activation and its downstream pro-inflammatory gene products, including cytokines, chemokines, and COX-2 [24].
The inverse relationship between TNF-α levels and tumor reduction further supports the role of inflammatory cytokines in promoting tumor growth, angiogenesis, and metastasis. This mechanism highlights curcumin’s ability to interrupt the tumor-promoting microenvironment, an essential aspect of its chemopreventive efficacy.
Tumor tissues often exhibit elevated oxidative stress, contributing to genetic instability and tumor progression. The significant reduction in MDA levels and enhancement of antioxidant enzymes (SOD, catalase) in turmeric-treated groups indicates effective oxidative stress mitigation. Curcumin is well-documented as a scavenger of reactive oxygen species (ROS), and this antioxidant capacity further contributes to its protective role against carcinogenesis [25, 26].
This study demonstrated a clear dose-response relationship, with the highest curative effects seen in the 400 mg/kg group. These doses were well-tolerated with no adverse behavioral or physiological effects observed, consistent with earlier toxicity studies that reported low systemic toxicity of curcumin even at high doses [27-31]. In conclusion, the present study demonstrates that Curcuma longa (turmeric) possesses potent therapeutic potential in the management of breast cancer in female rats. Oral administration of turmeric extract led to a significant and dose-dependent reduction in tumor volume, along with favorable modulation of molecular markers related to apoptosis (Caspase-3, p53), inflammation (TNF-α, NF-κB), and oxidative stress (MDA, SOD).
The observed effects are attributed primarily to curcumin, the principal bioactive component of turmeric, which exerted multifaceted anticancer effects by promoting programmed cell death, inhibiting pro-inflammatory pathways, and restoring redox balance. Notably, the highest dose (400 mg/kg) yielded the most pronounced therapeutic benefit, with no signs of systemic toxicity or adverse effects.
These findings suggest that turmeric, a widely available and well-tolerated natural compound, could serve as a promising complementary agent in breast cancer therapy, particularly for early-stage or hormone- responsive tumors. However, further investigations including mechanistic molecular studies, pharmacokinetic profiling, and clinical trials are warranted to validate these results and translate them into therapeutic applications for humans.
Acknowledgements
The authors would like to express their sincere gratitude to the Scientific Research Center, Al-Ayen Iraqi University, for providing the necessary facilities and support to carry out this research. Special thanks are extended to the technical staff and animal care personnel for their assistance in animal handling, histopathological preparations, and data collection.
We also acknowledge the contribution of [Insert Collaborator Name, if any], whose guidance and critical feedback significantly enriched the scientific rigor of this work.
This study was conducted as part of a non-commercial academic investigation. No external funding was received.
Conflict of Interest
The authors declare no conflict of interest related to the publication of this research. The study was conducted independently and received no financial support or sponsorship from commercial or institutional entities that could influence the results or interpretation of the data.
References
- Antiproliferative effect of curcumin (diferuloylmethane) against human breast tumor cell lines Mehta K., Pantazis P., McQueen T., Aggarwal B. B.. Anti-Cancer Drugs.1997;8(5). CrossRef
- Therapeutic efficacy of Curcuma longa extract on 7,12-dimethylbenz[a]anthracene-induced breast cancer in female Wistar rats El-Zein N, Abdelgawad M, Farouk SM , Abdallah AT , Saleh M. Environ Toxicol Pharmacol.2021;81:103510.
- Anticancer potential of curcumin: preclinical and clinical studies Aggarwal BB , Kumar A, Bharti AC . Anticancer Research.2003;23(1A).
- The multifaceted role of curcumin in cancer prevention and treatment Shanmugam MK , Rane G, Kanchi MM , Arfuso F, Chinnathambi A, Zayed M. E., Alharbi SA , et al . Molecules (Basel, Switzerland).2015;20(2). CrossRef
- Quantitative cellular uptake, localization and cytotoxicity of curcumin in normal and tumor cells Kunwar A., Barik A., Mishra B., Rathinasamy K., Pandey R., Priyadarsini K. I.. Biochimica Et Biophysica Acta.2008;1780(4). CrossRef
- The targets of curcumin Zhou H, Beevers CS , Huang S. Current Drug Targets.2011;12(3). CrossRef
- Curcumin blocks cytokine-mediated NF-kappa B activation and proinflammatory gene expression by inhibiting inhibitory factor I-kappa B kinase activity Jobin C., Bradham C. A., Russo M. P., Juma B., Narula A. S., Brenner D. A., Sartor R. B.. Journal of Immunology (Baltimore, Md.: 1950).1999;163(6).
- Therapeutic roles of curcumin: lessons learned from clinical trials Gupta SC , Patchva S, Aggarwal BB . The AAPS journal...2013;15(1). CrossRef
- Preparation and standardization of curcumin extract from Curcuma longa L. rhizome Gopi S, Jacob J, Varma K, Jude S, Raghu K, George R. Int J Pharm Sci Res.2016;7(8):3274-3279. CrossRef
- Dose escalation of a curcuminoid formulation Lao CD , Ruffin MT , Normolle D, Heath DD , Murray SI , Bailey JM , Boggs ME , et al . BMC complementary and alternative medicine.2006;6. CrossRef
- Incorporating of Cobalt into UiO-67 Metal–Organic Framework for Catalysis CO2 Transformations: An Efficient Bi-functional Approach for CO2 Insertion and Photocatalytic Reduction Al-dolaimy F., Kzar MH , Hussein SA , Bahir H, Hamoody AM , Dawood AH , Qasim MT , et al . Journal of Inorganic and Organometallic Polymers and Materials.2024;34(2). CrossRef
- The functions and molecular mechanisms of Tribbles homolog 3 (TRIB3) implicated in the pathophysiology of cancer Arif , Alameri AA , Tariq UB , Ansari SA , Sakr HI , Qasim MT , Aljoborae FFM , et al . International Immunopharmacology.2023;114. CrossRef
- Exosomal Non-coding RNA Derived from Mesenchymal Stem Cells (MSCs) in Autoimmune Diseases Progression and Therapy; an Updated Review Farhan SH , Jasim SA , Bansal P, Kaur H, Abed Jawad M, Qasim MT , Jabbar AM , et al . Cell Biochemistry and Biophysics.2024;82(4). CrossRef
- Double-edged sword role of miRNA-633 and miRNA-181 in human cancers Gupta J, Suliman M, Ali R, Margiana R, Hjazi A, Alsaab HO , Qasim MT . Pathology, Research and Practice.2023;248. CrossRef
- The pathological role of C-X-C chemokine receptor type 4 (CXCR4) in colorectal cancer (CRC) progression; special focus on molecular mechanisms and possible therapeutics Hjazi A, Nasir F, Noor R, Alsalamy A, Zabibah RS , Romero-Parra RM , Ullah MI , et ak . Pathology, Research and Practice.2023;248. CrossRef
- Unraveling the impact of 27-hydroxycholesterol in autoimmune diseases: Exploring promising therapeutic approaches Hjazi A, Ahsan M, Alghamdi MI , Kareem A. K., Al-Saidi DN DN, Qasim MT, Romero-Parra RM, et al . Pathology, Research and Practice.2023;248. CrossRef
- A comprehensive insight into the contribution of epigenetics in male infertility; focusing on immunological modifications Hsu CY , Jasim SA , Pallathadka H, Kumar A, Konnova K, Qasim MT , Alubiady MHS , et al . Journal of Reproductive Immunology.2024;164. CrossRef
- MicroRNA-enriched exosome as dazzling dancer between cancer and immune cells Hsu C, Ahmed AT , Bansal P, Hjazi A, Al-Hetty HRAK , Qasim MT , Sapaev I, et al . Journal of Physiology and Biochemistry.2024;80(4). CrossRef
- Tumor-associated Macrophages (TAMs) in Cancer Resistance; Modulation by Natural Products Lafta HA , AbdulHussein AH , Al-Shalah SAJ , Alnassar YS , Mohammed NM , Akram SM , Qasim MT , Najafi M. Current Topics in Medicinal Chemistry.2023;23(12). CrossRef
- Detection of abemaciclib, an anti-breast cancer agent, using a new electrochemical DNA biosensor Lei Z, Alwan M, Alamir HTA , Alkaaby HHC , Farhan SS , Awadh SA , Altimari US , et al . Frontiers in Chemistry.2022;10. CrossRef
- A comprehensive immunobiology review of IBD: With a specific glance to Th22 lymphocytes development, biology, function, and role in IBD Lv J, Ibrahim YS , Yumashev A, Hjazi A, Faraz A, Alnajar MJ , Qasim MT , et al . International Immunopharmacology.2024;137. CrossRef
- Functions and therapeutic interventions of non-coding RNAs associated with TLR signaling pathway in atherosclerosis Margiana R, Alsaikhan F, Al-Awsi grl , Patra I, Sivaraman R, Fadhil AA , Al-Baghdady HFA , et al . Cellular Signalling.2022;100. CrossRef
- Molecular identification of Cystoisospora belli in patients infected with the human immunodeficiency virus Qasim MT , Fenjan MN , Thijail HA . Int J Drug Deliv Technol.2022;12(2):701-704. CrossRef
- The association of Helicobacter pylori infection and virulence factors in gastric cancer in Thi-Qar, Iraq Qasim MT , Mohammed ZI . Asian Pac J Cancer Biol.2024;9(4):541-545. CrossRef
- Ovine Pasteurellosis Vaccine: Assessment of the Protective Antibody Titer and Recognition of the Prevailing Serotypes Qasim M. T., Mahdi Mohammed Alakkam E., Mahdi Mohammed M., Hachim S. K., Sabah Jabr H., Emad Izzat S., Mohammed K. A., et al . Archives of Razi Institute.2022;77(3). CrossRef
- Investigating treatment response and viral immunity in early rheumatoid arthritis via immune response profiling Qasim MT , Mohammed ZI . J Rare Cardiovasc Dis.2024;4(8):166-173.
- Investigating the effect of pregabalin on postoperative pain in non-emergency craniotomy Sane S, Mahoori A, Abdulabbas HS , Alshahrani SH , Qasim MT , Abosaooda M, Nozad P, et al . Clinical Neurology and Neurosurgery.2023;226. CrossRef
- Development of antihyperlipidemic drug-loaded β-CD-based microparticulate carrier systems: tuning and optimization Ul Hassan Shah Z, Abbasi A, Bukhari S, et al . Polym Plast Technol Mater.;63(11):1438-1463.
- Effects of Resveratrol on Nonmelanoma Skin Cancer (NMSC): A Comprehensive Review Zamanian MY , Shahbazi T, Kazmi SW , Hussien BM , Sharma A, Qasim MT , Hjazi A, et al . Food Science & Nutrition.2024;12(11). CrossRef
- The Impact of glomerulonephritis on cardiovascular disease: Exploring pathophysiological links and clinical implications Qasim, Maytham T, Zainab I. Mohammed . 5.2025;1:3-8.
- Exploring the Immunological and Physiological Effects of Zingiber Officinale on Women with Ovarian Cancer in Iraq Qasim, Maytham T, Lina A. Hameed , Zainab I. Mohammed . Asian Pacific Journal of Cancer Biology.2025;10(2):261-268. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details