The Ratio of MicroRNA-21 to MicroRNA-195 as a Predictive and Prognostic Biomarker of Colorectal Carcinoma
Download
Abstract
Introduction: Colorectal carcinoma (CRC) is the fourth leading cause of cancer-related death worldwide and the third most common cancer in Indonesia. Due to its asymptomatic nature, CRC is often diagnosed at an advanced stage, making early detection critical. MicroRNAs (miRNAs), small non-coding single-stranded RNA molecules, have emerged as potential biomarkers for cancer.
Objective: This study aimed to determine the ratio profile of miR-21 and miR-195 as predictive and prognostic factors in colorectal carcinoma patients in Margono Soekarjo Hospital, Purwokerto, Indonesia.
Methods: This study was conducted with a cross-sectional study design on 31 colorectal carcinoma patients during the 2018-2020 period who still had remaining paraffin-embedded tissue blocks at the Anatomical Pathology Laboratory of Margono Soekarjo Hospital, Purwokerto, Indonesia. The expression levels of miR-21 and miR-195 were analyzed using quantitative polymerase chain reaction (qPCR). The Pearson correlation test assessed the association between miRNA expression and clinicopathological parameters.
Result: This study revealed that high miR-21 expression levels were significantly associated with poor prognosis in colorectal carcinoma patients, with p values of 0.027 and r = 0.398. Low miR-195 expression levels were significantly associated with poor colorectal carcinoma prognosis, with p values of 0.002 and r=0.533. A high miR-21/miR-195 expression levels ratio is significantly associated with the advanced colorectal carcinoma stage, with p values of 0.016 and r = 0.429.
Conclusion: A High ratio of miR-21/ miR-195 correlates with the advanced stage of colorectal carcinoma. The expression ratio of miR-21 to miR-195 may be a potential predictive and prognostic biomarker in colorectal carcinoma.
Introduction
Colorectal carcinoma (CRC) is a malignancy originating from the lumen surface of the colon or rectum, with the most common type being adenocarcinoma [1, 2]. It ranks third after lung cancer and breast cancer, with 9.6 % of new cases and 9.3% of deaths [3].
Colorectal carcinoma is one of the most common malignancies in Western countries and the second leading cause of death from carcinoma in the United States [4]. According to the International Agency for Research on Cancer (IARC), in 2015, an estimated 9.7% of all cancers were CRC, with about 814.000 cases in men and 664.000 cases in women. Therefore, it makes CRC the third most common cancer in men and the second in women [5].
Cancer development requires exposure to carcinogens and accumulation of multiple mutations of tumor suppressor genes [6]. The role of microRNAs (miRs) has been found in the oncogenesis and progression of CRC, invasion, metastasis, and angiogenesis. The mechanism involves mutations, deletions, promoter methylation, or other abnormalities in miR biogenesis, leading to overexpression of oncogenic miRs [7-9].
Two miRs were found to be influential in the carcinogenesis of CRC, namely miR-21 and miR-195. miR-21 has the property of being an oncogene [7]. In contrast, miR-195 has the property of being a tumor suppressor, where miR-195 suppresses cell proliferation, colony formation of cancer cells, and invasion of cancer cells [8].
This study aims to determine the ratio profile between miR-21 and miR-195 as predictive and prognostic biomarkers in colorectal carcinoma at Margono Soekarjo Hospital, Purwokerto. During our research, no study has looked for the profile of the ratio between miR-21 and miR-195 in colorectal carcinoma at Margono Soekarjo Hospital, Purwokerto.
Materials and Methods
This study was conducted with a cross-sectional study design. The target population in this study was all patients with a diagnosis of colorectal carcinoma. The affordable population in this study was all colorectal carcinoma patients at Margono Soekarjo Hospital during the 2018- 2020 period who still had paraffin block tissue left in the Anatomical Pathology Laboratory. Study participants were selected based on specific inclusion and exclusion criteria. The inclusion criteria consisted of patients with a confirmed diagnosis of colorectal carcinoma and availability of complete medical records that included both tumor staging and histopathological grading data. The exclusion criteria included patients who had previously undergone any form of therapy for colorectal carcinoma, as well as cases with an uncertain or unconfirmed diagnosis. Written informed consent was obtained from all patients or their legal guardians before data collection and tissue utilization. To ensure patient confidentiality, all clinical data were anonymized before analysis.
Deparafinisation
Sections of 20μm were cut from Formalin-fixed paraffin-embedded (FFPE) tissue blocks, and up to 80 μm of tissue slices were placed in a microcentrifuge tube. The samples were treated with xylene, heated at 50°C to melt the paraffin, and centrifuged. After removing the xylene, the tissue was washed twice with 100% ethanol, centrifuged, and the ethanol was discarded. Finally, the pellet was air-dried for 15–30 minutes to remove residual ethanol, preparing the tissue for analysis [9].
Protease Digestion
The digestion buffer and protease were added to each sample, followed by gentle mixing to ensure complete tissue immersion. The samples were then incubated for 3 hours at 50°C. If the mixture remained unclear after incubation, the undigested tissue was retained and not removed during filtration. Samples were subsequently stored at −20°C and thawed on ice before proceeding with total RNA isolation [9].
RNA extraction
An isolation additive was added to each sample, followed by vortexing until the solution appeared white and cloudy. Ethanol was added, and the solution was mixed by pipetting until it became clear. Each sample was carefully applied to a filter cartridge placed in a collection tube, ensuring large tissue fragments were avoided. The mixture was passed through the filter using centrifugation, and the flow-through was discarded after each spin. This process was repeated until the entire sample mixture had been filtered. The filter was washed twice with Wash 1 and 2 solutions, followed by centrifugation and disposal of the flow-through after each wash. A final spin was performed to eliminate any residual liquid from the filter [9].
cDNA Synthesis
Twenty nanograms of RNA were reverse transcribed into complementary DNA (cDNA) in a final volume of 10 µL, utilizing the Universal cDNA Synthesis Kit. The resulting cDNA was divided into 2 µL aliquots and stored at -80 °C for future use. To ensure precision in the method’s setup and analysis, cDNA synthesis was carried out in duplicate, with 20 ng of RNA used for each reaction [10].
Real-time PCR procedure to quantify miR-21 and miR- 95 expression
Quantitative reverse transcription PCR (qRT-PCR) was performed to analyze miR-21, miR-195, and RNU6-2 expression levels using the Gene Hyperscript RT-PCR MasterMix ExiLent STBR according to the manufacturer’s instructions. Complementary DNA (cDNA) was synthesized from total RNA using a universal cDNA synthesis master mix. Each PCR reaction was conducted in a total volume of 25 μL containing 1× QuantiTect SYBR Green PCR Master Mix (Qiagen), 0.5 μM of each specific forward and reverse primer, one μL of cDNA, and nuclease-free water to complete the volume. The primers used were as follows: miR-195 forward 5′-ACACTCCAGCTGGGTAGCAGCACAGAAAT-3′ and reverse 5′-TGGTGTCGTGGAGTCG-3′; m i R - 2 1 f o r w a r d 5′-ACACTCCAGCTGGGTAGCTTATCAGACTGAT-3′ and reverse 5′-ACTGGTGTCGTGGAGTCG-3′; and RNU6-2 (used as an endogenous control) forward 5′-CTCGCTTCGGCAGCACA-3′ and reverse 5′-AACGCTTCACGAATTTGCGT-3′. The thermal cycling conditions included an initial denaturation at 95°C for 5 minutes, followed by 40 cycles of 94˚C for 30 sec, 65˚C for 30 sec, and 72˚C for 30 sec. All reactions were run in technical triplicate to ensure the reliability and reproducibility of the results. The relative gene expression was analyzed using the ΔΔCt method.
Data analysis
The correlation between the miR-21/miR-195 expression ratio and clinicopathological parameters was evaluated using the Pearson correlation coefficient for normally distributed data. A p-value of less than 0.05 was considered statistically significant.
Results
Characteristics of the research subject
The sample characteristics included sex and TNM staging of CRC [11]. The sample consisted of slightly equal males and females. Most of the patients were in CRC stage 3, followed by stage 2, stage 4, and stage 1 (Table 1).
| Stage 1 | Stage 2 | Stage 3 | Stage 4 | |
| N (%) | 3 (9.67) | 10 (32.26) | 14 (45.17) | 4 (12.9) |
| Gender (M/F) | (3/0) | (4/6) | (7/7) | (2/2) |
| T stages | ||||
| T2 | 3 | 1 | 0 | 0 |
| T3 | 0 | 4 | 4 | 0 |
| T4 | 0 | 5 | 10 | 4 |
| N stages | ||||
| N0 | 3 | 10 | 0 | 0 |
| N1 | 0 | 0 | 4 | 3 |
| N2 | 0 | 0 | 10 | 1 |
| M stages | ||||
| M0 | 3 | 10 | 13 | 0 |
| M1 | 0 | 0 | 1 | 4 |
TNM classification based on the International Union against Cancer
miR-21 expression levels and stage of colorectal carcinoma
The expression level of miR-21 increased progressively with advancing colorectal cancer stage. The lowest expression was observed in stage 1 (9.04 ± 0.77), followed by stage 2 (22.74 ± 2.47), stage 3 (17.99 ± 2.76), and the highest in stage 4 (34.50 ± 2.18), as shown in Figure 1A.
Figure 1. Bar Diagram of miR-21 Expression Level (A) and miR-195 Expression Level (B) at each Stage of CRC.
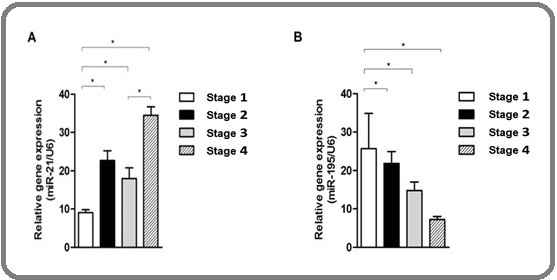
Pearson correlation analysis revealed a moderate positive correlation between miR-21 expression and cancer stage (r = 0.398, p = 0.027) (Table 2), indicating that higher miR-21 levels are associated with more advanced disease.
| Parameters | Coefficient Value | |
| R | P | |
| Gender | 0.14 | 0.451 |
| Primary Tumor (T) | 0.644 | <0.001* |
| Regional Lymph Node (N) | 0.712 | <0.001* |
| Distant Metastases (M) | 0.627 | <0.001* |
| miR-21 | 0.398 | 0.027* |
| miR-195 | -0.533 | 0.002* |
| miR-21/miR-195 ratio | 0.429 | 0.016* |
*p-value <0.05 is judged as significant; r = coefficient of correlation
The statistically significant differences across several stages (p < 0.05) support the role of miR-21 as a potential oncogenic biomarker involved in tumor progression and staging in colorectal carcinoma.
miR-195 expression levels and stage of colorectal carcinoma
In contrast to miR-21, miR-195 expression levels decreased progressively with advancing colorectal cancer stage. The highest expression was observed in stage 1 (25.67 ± 9.17), followed by stage 2 (21.85 ± 3.09), stage 3 (14.83 ± 2.16), and the lowest in stage 4 (7.23 ± 0.82), as shown in Figure 1B. Pearson correlation analysis revealed a moderate negative correlation between miR-195 expression and cancer stage (r = –0.533, p = 0.002) (Table 2), which is associated with more progressive tumor staging in colorectal carcinoma. This statistically significant downward trend supports the role of miR-195 as a putative tumor suppressor, whose reduced expression may contribute to colorectal cancer progression.
miR-21/miR-195 expression levels ratio and stage of colorectal carcinoma
In this study, the miR-21/miR-195 expression ratio showed a significant positive correlation with colorectal cancer stage (r = 0.429, p = 0.016; Table 2), indicating that a higher ratio is associated with more progressive tumor staging in colorectal carcinoma. This trend reflects the combined effect of increasing miR-21 and decreasing miR-195 expression as the malignancy progresses. These findings support the potential utility of the miR-21/ miR-195 ratio as a composite molecular biomarker for tumor staging and progression assessment in colorectal carcinoma.
Discussion
This study found that the greater the level of miR-21 expression, the higher the stage of colorectal carcinoma. This result aligns with previous research that stated that high levels of miR-21 expression have a significant relationship with tumor size, distant metastases, and poor life expectancy [12, 13]. It happens because the expression of miR-21, an oncogenic microRNA, increases along with the progression of tumor size. Serum miR-21 expression levels were higher in patients with colorectal carcinoma than in adenoma patients [14]. This high level of miR-21 in colorectal carcinoma supports miR-21 as a potential biomarker for detecting and assessing the prognosis of colorectal carcinoma. A study compared miR-21, CEA, and CA 19-9 in diagnosing colorectal carcinoma with a sensitivity of 61.4%, 30.7%, and 15.9%, respectively [15]. This finding emphasizes the potential superiority of miR-21 as a non-invasive biomarker, particularly in early-stage detection, where traditional markers often lack sensitivity. Unlike CEA and CA 19-9, which are frequently elevated only in advanced disease, miR-21 expression has been consistently shown to increase even in precancerous lesions and early-stage CRC [16]. Cut-off values for miR-21 vary depending on sample type and clinical purpose. For CRC diagnosis, reported thresholds include a 1.961-fold increase (64% sensitivity, 68% specificity), ≥5.25 in serum (94.3% sensitivity, 93.3% specificity), and >6.44 to distinguish CRC from benign colorectal conditions [17–19]. The biological relevance of miR-21 in CRC progression can be further understood through its underlying molecular mechanisms. miR-21 promotes tumorigenesis by downregulating several tumor suppressor genes, including phosphatase and tensin homolog (PTEN), PDCD4, RECK, and RhoB small interfering RNA (si-RhoB), which leads to activation of the PI3K/AKT pathway, increased cell proliferation, resistance to apoptosis, and enhanced invasiveness through upregulation of MMP-2/9 [20]. Additionally, miR-21 contributes to a pro-inflammatory tumor microenvironment by upregulating COX-2 (PTGS2) and its downstream effector, PGE2, while suppressing PGE2- degrading enzymes [21]. It also interferes with cell cycle regulation by targeting Cdc25A, disrupting G1/S and G2/M checkpoints. Furthermore, miR-21 impairs TGF-β signaling by downregulating TGFβR2, enhancing cancer cell stemness and invasiveness [22, 23].
We found that the lower the level of miR-195 expression, the higher the stage of colorectal carcinoma, indicating its potential role as a tumor suppressor. This finding is consistent with previous studies demonstrating that miR–195–5p downregulation facilitates colorectal cancer progression through multiple oncogenic mechanisms [24]. Mechanistically, miR–195–5p is known to suppress several critical signaling pathways involved in colorectal carcinogenesis. It negatively regulates the Wnt/β-catenin pathway by targeting components such as Axin2 and Frizzled-4 (FZD4); its downregulation leads to activation of this pathway and enhanced tumor proliferation and invasion [23, 25]. Beyond the Wnt pathway, miR–195–5p also exerts tumor-suppressive functions through other oncogenic pathways. It is downregulated by lncRNAs such as NEAT1 and AFAP1-AS1, which function as competing endogenous RNAs (ceRNAs), resulting in the overexpression of oncogenes like CEP55 and WISP1 that promote tumor growth, migration, and resistance to apoptosis [26]. Furthermore, miR–195–5p inhibits the Hippo/YAP pathway by targeting YAP1, thereby preventing epithelial-mesenchymal transition (EMT) and metastatic progression. Reduced miR–195–5p expression also increases GDPD5 levels, facilitating glycolysis, chemoresistance, and metastatic potential. Within the tumor microenvironment, miR–195–5p modulates immune responses by suppressing the Notch2/GATA3/ IL-4, reducing the infiltration of M2-like tumor-associated macrophages, and limiting cancer cell stemness [27]. Previous studies have shown that decreased levels of miR-195 expression are significantly associated with colorectal carcinoma patients with advanced lymph node metastases and stages [28, 29]. In addition, it is also related to lower patient life expectancy. miR-195 is a tumor suppressor gene in which miR-195 downregulation occurs in the progression of colorectal carcinoma. Notably, a study proposed a cut-off value of ≤0.488 for miR-195 expression, below which the risk of poor prognosis and progression is significantly increased, supporting its utility as a prognostic biomarker [30]. The decrease in miR-195 causes tumor progression, angiogenesis, and tumor invasion to increase, so that the stage of carcinoma is higher. Previous research has also mentioned that miR- 195 can predict life expectancy in patients with colorectal carcinoma [28, 30, 31].
A high miR-21/miR-195 ratio is related to a higher stage of colorectal carcinoma. To our knowledge, no studies have studied this ratio, and most previous studies have used serum and feces samples from healthy patients and controls. MiR-21 is an oncogenic gene that plays a role in tumor progression, while miR-195 plays a role in the tumor suppressor gene that inhibits the rate of tumor progression [17, 28]. So, when there is a high miR-21/ miR-195 ratio, it can be estimated that it is related to the further stage of carcinoma. Further research needs to be carried out to confirm the results obtained to support the findings of the researchers further.
This study is subject to several limitations. First, the relatively small sample size (n = 31) may have reduced the statistical power and limited the generalizability of the results. The sample size was determined by the number of eligible cases with available archival tissue that met the inclusion criteria during the specified data collection period. As a result, a formal a priori power analysis was not conducted. Second, potential tissue heterogeneity may have influenced the observed miRNA expression levels. Although standardized protocols were applied for sample preparation and processing, variations in cellular composition across formalin-fixed paraffin-embedded (FFPE) tissues could not be entirely controlled. Such heterogeneity may introduce bias and obscure actual biological differences. Future research should involve larger, more diverse patient cohorts and incorporate prospective study designs to strengthen these findings. In addition, applying cell-type-specific isolation techniques or single-cell analyses may help reduce tissue complexity and provide more accurate insights into the role of miRNAs in colorectal cancer progression.
In conclusion, the study evaluated the expression of the miR-21/miR-195 ratio in colorectal carcinoma tissue as a potential predictive and prognostic factor in patients treated at Margono Soekarjo Hospital, Purwokerto. The results demonstrated a significant association between tissue miR-21 expression and colorectal carcinoma prognosis, with a P-value of 0.027 (P<0.05) and a correlation coefficient (r) of 0.398, indicating that higher miR-21 expression corresponds to more advanced cancer stages. Similarly, tissue miR-195 expression showed a significant inverse correlation with prognosis (P=0.002, r=-0.533), suggesting that lower miR-195 expression is associated with more advanced stages of colorectal carcinoma. Furthermore, the study confirmed the hypothesis that an increased miR-21/miR-195 ratio correlates with higher stages of colorectal carcinoma, as indicated by a P-value of 0.016 (P<0.05) and a correlation coefficient of 0.429. These findings support the potential role of the miR-21/ miR-195 ratio as a predictive and prognostic biomarker in colorectal carcinoma.
Conflict of Interest
The authors declare there is no conflict of interest.
Ethical Declaration
This study was approved by the ethical committee of the Faculty of Medicine, Universitas Jenderal Soedirman, Purwokerto, Indonesia.
References
- Colorectal carcinoma: Pathologic aspects Fleming M, Ravula S, Tatishchev SF , Wang HL . Journal of Gastrointestinal Oncology.2012;3(3). CrossRef
- Colorectal Carcinoma: A General Overview and Future Perspectives in Colorectal Cancer Mármol I, Sánchez-de-Diego C, Pradilla Dieste A, Cerrada E, Rodriguez Yoldi MJ . International Journal of Molecular Sciences.2017;18(1). CrossRef
- Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL , Soerjomataram I, Jemal A. CA: a cancer journal for clinicians.2024;74(3). CrossRef
- An update on miRNAs as biological and clinical determinants in colorectal cancer: a bench-to-bedside approach Weng W, Feng J, Qin H, Ma Y, Goel A. Future Oncology (London, England).2015;11(12). CrossRef
- Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012 Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM , Forman D, Bray F. International Journal of Cancer.2015;136(5). CrossRef
- Tumor Suppressors Having Oncogenic Functions: The Double Agents Datta N, Chakraborty S, Basu M, Ghosh MK . Cells.2020;10(1). CrossRef
- Apoptosis and the target genes of microRNA-21 Buscaglia LEB , Li Y. Chinese Journal of Cancer.2011;30(6). CrossRef
- MicroRNA-195 inhibits colorectal cancer cell proliferation, colony-formation and invasion through targeting CARMA3 Wang L, Qian L, Li X, Yan J. Molecular Medicine Reports.2014;10(1). CrossRef
- MicroRNA isolation from formalin-fixed, paraffin-embedded tissues Liu A, Xu X. Methods in Molecular Biology (Clifton, N.J.).2011;724. CrossRef
- Reliability of miRNA Analysis from Fixed and Paraffin-Embedded Tissues Azzalini E, De Martino E, Fattorini P, Canzonieri V, Stanta G, Bonin S. International Journal of Molecular Sciences.2019;20(19). CrossRef
- American Cancer Society. American Cancer Society. 2024. Colorectal Cancer Early Detection, Diagnosis, and Staging. Available from: https://seer.cancer.gov/csr/1975_2016/ .
- miR-21: a promising biomarker for the early detection of colon cancer Dehghan F, Boozarpour S, Torabizadeh Z, Alijanpour S. OncoTargets and Therapy.2019;12. CrossRef
- Diagnostic role of circulating MiR-21 in colorectal cancer: a update meta-analysis Liu T, Liu D, Guan S, Dong M. Annals of Medicine.2021;53(1). CrossRef
- Serum miR-21 as a diagnostic and prognostic biomarker in colorectal cancer Toiyama Y, Takahashi M, Hur K, Nagasaka T, Tanaka K, Inoue Y, Kusunoki M, Boland CR , Goel A. Journal of the National Cancer Institute.2013;105(12). CrossRef
- Considering Exosomal miR-21 as a Biomarker for Cancer Shi J. Journal of Clinical Medicine.2016;5(4). CrossRef
- A plasma microRNA panel for detection of colorectal adenomas: a step toward more precise screening for colorectal cancer Kanaan Z, Roberts H, Eichenberger MR , Billeter A, Ocheretner G, Pan J, Rai SN , et al . Annals of Surgery.2013;258(3). CrossRef
- Potential Values of Circulating microRNA-21 to Predict Early Recurrence in Patients with Colorectal Cancer after Treatments Hao Y, Yang C, Chen M, Chang L, Lin C, Lo L, Huang S, et al . Journal of Clinical Medicine.2022;11(9). CrossRef
- Role of serum miR-21 and miR-92a in colorectal cancer diagnosis as novel molecular biomarkers Hassan R, Omar M, Shehata M, Raafat M, Hamdy A, Zedan A, et al . International Journal of Cancer and Biomedical Research.2021;5(1):95-104.
- Evaluation of the Role of miRNA-21 Levels as a Potential Diagnostic Biomarker for Colorectal Cancer Associated with Prognosis Falih ES , Obaid SH , Hameed FR . Medico-legal Update.2022;20(2).
- Roles of miR-21 in the Onset and Advancement of Colorectal Cancer (CRC) Kordkatouli M, Mohammadi bondarkhilli S, Sateei A, Mahmood Janlou MA . Multidisciplinary Cancer Investigation.2024;8(1). CrossRef
- MicroRNA MIR21 (miR-21) and PTGS2 Expression in Colorectal Cancer and Patient Survival Mima K, Nishihara R, Yang J, Dou R, Masugi Y, Shi Y, Silva A, et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2016;22(15). CrossRef
- A circRNA-miRNA-mRNA network identification for exploring underlying pathogenesis and therapy strategy of hepatocellular carcinoma Xiong D, Dang Y, Lin P, Wen D, He R, Luo D, Feng Z, Chen G. Journal of Translational Medicine.2018;16(1). CrossRef
- MicroRNA-21 is immunosuppressive and pro-metastatic via separate mechanisms Chi LH , Cross RSN , Redvers RP , Davis M, Hediyeh-Zadeh S, Mathivanan S, Samuel M, et al . Oncogenesis.2022;11(1). CrossRef
- Roles and mechanisms of miR-195-5p in human solid cancers Xu Q, Xu J, Chen W, Xu W, Song Y, Tang W, Xu D, Jiang M, Tang J. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie.2022;150. CrossRef
- CircRASSF2 facilitates the proliferation and metastasis of colorectal cancer by mediating the activity of Wnt/β-catenin signaling pathway by regulating the miR-195-5p/FZD4 axis Yang L, Bi T, Zhou S, Lan Y, Zhang R. Anti-Cancer Drugs.2021;32(9). CrossRef
- Evaluation of Potential of Long Noncoding RNA NEAT1 in Colorectal Cancer Cheng H, Malhotra A. Journal of Environmental Pathology, Toxicology and Oncology: Official Organ of the International Society for Environmental Toxicology and Cancer.2020;39(2). CrossRef
- GDPD5, a target of miR-195-5p, is associated with metastasis and chemoresistance in colorectal cancer Feng C, Zhang L, Sun Y, Li X, Zhan L, Lou Y, Wang Y, Liu L, Zhang Y. Biomedicine & Pharmacotherapy = Biomedecine & Pharmacotherapie.2018;101. CrossRef
- Downregulation of miR-195 correlates with lymph node metastasis and poor prognosis in colorectal cancer Wang X, Wang J, Ma H, Zhang J, Zhou X. Medical Oncology (Northwood, London, England).2012;29(2). CrossRef
- Comparative microRNA signatures based on liquid biopsy to identify lymph node metastasis in T1 colorectal cancer patients undergoing upfront surgery or endoscopic resection Okamoto K, Nozawa H, Ozawa T, Yamamoto Y, Yokoyama Y, Emoto S, Murono K, et al . Cell Death Discovery.2025;11(1). CrossRef
- MicroRNA Expression Profiling Predicts Nodal Status and Disease Recurrence in Patients Treated with Curative Intent for Colorectal Cancer Davey MG , Feeney G, Annuk H, Paganga M, Holian E, Lowery AJ , Kerin MJ , Miller N. Cancers.2022;14(9). CrossRef
- Integrated analysis identifies microRNA-195 as a suppressor of Hippo-YAP pathway in colorectal cancer Sun M, Song H, Wang S, Zhang C, Zheng L, Chen F, Shi D, et al . Journal of Hematology & Oncology.2017;10(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details