Improved Antitumor Efficacy of Liposome-Encapsulated Selenium Nanoparticles
Download
Abstract
Overview: This investigation chronicles the phytogenic synthesis of selenium nanoparticles (SeNPs) and their subsequent entrapment within phospholipid liposomes to construct a precision nanovector for oral-squamouscell-carcinoma therapy.
Methods: The plant-derived SeNPs were loaded into liposomes via a thin-film hydration approach. Dynamic light scattering (DLS) assessed their hydrodynamic diameter and zeta potential.
Results: This nanoparticle was then sequestered via a thin-film-hydration protocol that yielded liposomes with a mean hydrodynamic diameter of 235 nm, a polydispersity index of 0.15. Dynamic-release profiling in phosphate-buffered saline (pH 7.4, 37 °C) revealed a sustained discharge of 35 % of the payload over 62 h dramatically slower than the 95 % burst exhibited by free SeNPs attesting to the kinetic moderation conferred by the bilayer matrix. Functionally, MTT assays on an oral-cancer cell line demonstrated a 72 % reduction in viability after 24 h, significantly eclipsing the 38 % inhibition achieved by unencapsulated nanoparticles (p < 0.001).
Conclusion: These data indicate that liposomal sequestration furnishes SeNPs with enhanced colloidal stability, protracted release dynamics, and markedly elevated in-vitro antineoplastic potency, thereby positioning the platform as a compelling, biocompatible candidate for targeted oral-cancer therapeutics and warranting subsequent in-vivo validation.
Introduction
Today, a broad spectrum of scientific fields from biotechnology, nanotechnology, and materials science to machine learning, environmental engineering, and climate research are working together to solve complex challenges. This interdisciplinary convergence is delivering smart drugs and targeted delivery systems for cancer therapy, biocompatible materials for oral and bone tissue regeneration, optimized clean-energy Stirling engines, and high-resolution monitoring of natural hazards such as droughts and earthquakes. The result is better medical and dental care, higher industrial efficiency, greater environmental sustainability, and safer infrastructure evidence that the frontiers of knowledge are being harnessed to improve everyday life and drive future technologies across multiple domains [1-17]. Drawing on these studies, the deployment of cutting-edge technologies, artificial-intelligence algorithms, and advanced laboratory instrumentation has simultaneously boosted the safety and efficiency of civil infrastructure, enabled more accurate natural-hazard forecasting, and within medicine and dentistry leveraged advanced biomaterials, 3-D printing, and convolutional neural networks to improve implant design, deliver anticancer drugs with precision, and regenerate hard and soft tissues.
In parallel, AI‐driven analytics combined with temperature and pressure sensors are optimizing clean-energy systems and enhancing water-level and drought monitoring. This interdisciplinary convergence not only raises industrial efficiency and sustainability, but also provides society with more precise treatments, faster diagnoses, and safer infrastructure. Put simply, state-of-the-art techniques and devices are forging a seamless link between industry, medicine, and dentistry, charting new horizons for well-being and sustainable development [15, 18, 19-26]. The collective findings of these studies make it clear that the unprecedented pace of innovation in advanced technologies from artificial intelligence, deep learning, and bio-data analytics to nano-engineering, biotechnology, and micro-/nano-scale devices is redefining the frontiers of medical science and its allied fields. This interdisciplinary convergence is opening new horizons for faster, more accurate diagnostics, personalized therapies, and intelligent tissue-repair systems, while simultaneously charting a path toward better disease prevention, continuous health monitoring, and overall improvements in quality of life. Achieving this vision will demand deeper insight into biological mechanisms, the creation of novel computational methods, and the deployment of cutting-edge laboratory infrastructure [27-35]. Cancer is a complex, multifaceted disease that embraces many malignancies, notably colorectal cancer, esophageal cancer, and oral cancer [36-42]. For cancer treatment, liposomes and niosomes are the most widely used carriers thanks to their biocompatibility and targeting ability, whereas metallic (gold, selenium) and polymeric (PBCA) nanoparticles are also employed to optimize drug release, minimize side effects, and enhance therapeutic efficacy [43-50]. Selenium nanoparticles (SeNPs) have attracted considerable attention as antineoplastic agents due to their low inherent toxicity, antioxidative properties, and capacity to induce apoptosis selectively in cancer cells [51-53]. Moreover, green or “phytogenic” synthesis of SeNPs using plant-derived reducing extracts offers an environmentally benign and scalable route to biocompatible nanomaterials, often yielding particles with favorable stability and surface functionality [54, 55]. Nonetheless, unmodified SeNPs may still undergo premature clearance or aggregation in biological fluids, limiting their in-vivo efficacy [55, 56]. Liposomal encapsulation represents a well-established strategy to address these challenges. Phospholipid bilayers can entrap hydrophilic and hydrophobic payloads alike, provide steric stabilization, and afford tunable release profiles through bilayer composition and surface modification [57]. Liposomal formulations have been clinically validated in several oncological indications, demonstrating improved pharmacokinetics and reduced systemic toxicity [57]. By sequestering phytogenic SeNPs within liposomal carriers, it is possible to harness the cytocompatibility and antitumor activity of elemental selenium while optimizing nanoparticle stability, circulation time, and payload delivery [56, 58). we report the design, formulation, and in-vitro evaluation of a liposome-encapsulated SeNP platform tailored for OSCC treatment. We describe the green synthesis of SeNPs, their incorporation into phospholipid liposomes via thin-film hydration, and characterization of particle size, zeta potential, and release kinetics. Finally, we demonstrate the enhanced cytotoxic efficacy of the hybrid nanocarrier against an OSCC cell line, establishing a foundation for future in-vivo studies and potential translation into precision oral-cancer therapeutics.
Materials and Methods
Materials
Lecithin, cholesterol, polyethylene glycol 400 (PEG 400), as well as organic solvents including ethanol, isopropanol, and dimethyl sulfoxide (DMSO), were procured from Sigma-Aldrich (St. Louis, MO, USA). Essential cell culture reagents such as RPMI 1640, Dulbecco’s Modified Eagle Medium (DMEM), phosphate- buffered saline (PBS), fetal bovine serum (FBS), the tetrazolium-based cell viability reagent MTT, and a 100× Penicillin–Streptomycin antibiotic solution were sourced from Gibco (Thermo Fisher Scientific, USA). The human oral squamous cell carcinoma (OSCC) cell line HSC-3 was supplied by the National Cell Bank of Iran (NCBI), affiliated with the Pasteur Institute of Iran, following standard authentication and quality control protocols.
Green Biosynthesis of Selenium Nanoparticles (SeNPs)
Fresh aerial parts of Trifolium cherleri were sourced from the Iran Biological Resources Center, documented under herbarium accession code 1368, and taxonomically authenticated by a certified botanist. The collected plant material was air-dried in shaded, ventilated conditions to minimize oxidative and photolytic degradation. Once fully dried, it was ground using a laboratory-scale mechanical grinder to obtain a uniform, fine powder. For aqueous extraction, exactly 25 g of the powdered plant was infused in 120 mL of deionized water, followed by 24-hour maceration at ambient temperature to maximize the release of phytoconstituents. The mixture was subsequently filtered through Whatman filter paper (Merck, Germany) to remove insoluble residues, yielding a clear herbal extract. Selenium nanoparticles were synthesized via eco-friendly reduction using sodium selenite (Na₂SeO₃) as the precursor. A 1 mM solution of sodium selenite (250 mL) was prepared freshly under sterile laboratory conditions. To this solution, 20 mL of the clarified plant extract was added dropwise with gentle stirring at room temperature. A visible change in color ranging from pale yellow to orange-red served as a qualitative indicator of nanoparticle formation. The SeNPs were purified through repeated centrifugation at 12,000 rpm for 10 minutes, followed by triple washing with deionized water to remove residual phytochemicals and unreacted salts. Finally, the purified nanoparticles were freeze-dried and stored in moisture-free vials for further analyses and biomedical experimentation.
Fabrication of Liposome-Encapsulated Selenium Nanoparticles
Encapsulation of SeNPs into liposomes was carried out using the thin-film hydration technique. Specifically, 110 mg of lecithin, 60 mg of cholesterol, and 10 mg of PEG 400 were dissolved in 8 mL of chloroform in a sterile glass vial. The lipid solution was vortexed thoroughly and subjected to rotary evaporation at 65°C and 140 rpm to evaporate the solvent and form a consistent thin lipid film on the inner wall of the evaporation flask. To hydrate the dried lipid layer, 8 mL of an aqueous SeNP dispersion (concentration: 0.8 mg/mL) prepared in sterile phosphate-buffered saline (PBS) was added. The hydration step was carried out under mild heating (60°C) with continuous rotation at 100 rpm for 25 minutes, promoting the spontaneous assembly of lipid bilayers around the nanoparticles. The resulting multilamellar vesicles were processed by probe sonication for 4 minutes at 40% amplitude to produce a homogenous suspension of nanoscale liposomes. The final formulation was stored at 4°C for subsequent characterization studies focused on physicochemical stability and therapeutic performance.
Characterization of Nanoparticles
The average particle size, zeta potential, and polydispersity index (PDI) of the prepared nanoparticles were measured using dynamic light scattering (DLS) with the aid of a Nano ZS3600 Zetasizer (Malvern Instruments Ltd., UK), providing insights into their colloidal stability and size distribution.
In Vitro Drug Release Evaluation
The release behavior of selenium nanoparticle (SeNP) formulations was investigated using a dialysis-based diffusion method designed to mimic physiological conditions. Briefly, 2 mL of liposomal SeNPs and an equal volume of non-encapsulated (free) SeNPs were transferred into separate dialysis membranes (molecular weight cutoff: 12 kDa). Each dialysis bag was submerged in 20 mL of phosphate-buffered saline (PBS, pH 7.4) and maintained at 37 °C using a thermostatically controlled water bath. To facilitate uniform diffusion, the containers were placed on a magnetic stirrer with constant agitation throughout the experiment. At predetermined intervals (2, 5, 14, 22, 38, 56, and 62 hours), 2 mL aliquots were withdrawn from the external PBS medium and replaced immediately with an equal volume of pre-warmed fresh PBS to maintain sink conditions and ensure sustained drug release. The amount of released SeNPs in each sample was quantified by measuring absorbance at 265 nm using a UV–visible spectrophotometer. The cumulative release percentages were calculated and plotted over time, enabling direct comparison between the release kinetics of the liposome-encapsulated and free SeNPs formulations under identical conditions.
Assessment of Cytotoxicity
To evaluate the cytotoxic potential of selenium nanoparticles (SeNPs) in various formulations, including liposome-encapsulated SeNPs, free SeNPs, and unloaded liposomes, an MTT-based colorimetric assay was conducted on the HSC-3 oral squamous carcinoma cell line. Cells were seeded in 96-well plates at an initial density of 1 × 10⁴ cells per well and allowed to adhere and proliferate for 24 hours under standard culture conditions. Following this incubation, the cells were exposed to a range of concentrations (3.5 to 896 µg/ mL) of both liposomal and free SeNP formulations. After another 48-hour incubation period post-treatment, MTT reagent (5 mg/mL prepared in PBS) was added to each well and allowed to incubate for an additional 3 hours to facilitate the formation of formazan crystals. Subsequently, the medium was carefully aspirated, and 100 µL of DMSO was added to solubilize the crystals formed by metabolically active cells. Absorbance was recorded at 540 nm using a microplate spectrophotometer. Cell viability was calculated as a percentage relative to the untreated control group using the formula below:
Cell viability (%) = (Absorbance of treated cells / Absorbance of control cells) × 100
Statistical Analysis
All experiments were conducted in triplicate to ensure reproducibility. Data were analyzed using GraphPad Prism (version 8), employing one-way analysis of variance (ANOVA) for comparison among groups. A p-value of less than 0.05 was considered statistically significant.
Results
Green Synthesis and Characterization of SeNPs and Liposomal Formulations
To initiate the biosynthesis process, Trifolium cherleri powder was soaked in an aqueous medium for 24 hours to enable the efficient extraction of phytochemicals. The resulting extract was subsequently clarified through filtration using Whatman-grade filter paper. Upon gradual addition of the plant extract to a pre-prepared sodium selenite (Na₂SeO₃) solution at room temperature, a visible shift in color from light yellow to reddish-orange was observed, indicating the successful bioreduction of selenium ions and the formation of selenium nanoparticles (SeNPs). The selenium nanoparticles were encapsulated using the thin-film hydration technique, resulting in liposomal formulations with an average hydrodynamic diameter of 235 ± 12 nm, a polydispersity index (PDI) of 0.15 ± 0.02, and a zeta potential of approximately –28.6 ± 1.7 mV, indicating good colloidal stability and narrow size distribution (Table 1).
| Parameter | Measured Value | Unit |
| Mean Hydrodynamic Diameter | 235 ± 12 | nm |
| Polydispersity Index (PDI) | 0.15 ± 0.02 | — |
| Zeta Potential | –28.6 ± 1.7 | mV |
Zeta Potential –28.6 ± 1.7 mV
Drug release evaluation
Figure 1 depicts the cumulative release behavior of free selenium nanoparticles (SeNPs) versus those encapsulated within liposomes over a 62-hour period in phosphate-buffered saline (PBS, pH 7.4), simulating physiological conditions.
Figure 1.The Release Profile of SeNPs Encapsulated in Liposomes at 62 Hours. Data are expressed as mean ± SD from three independent experiments.
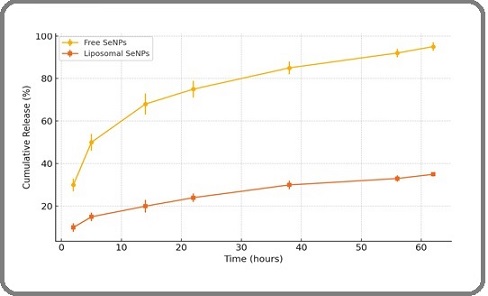
The results reveal a markedly slower release profile for liposomal SeNPs, with only approximately 35% of the payload released by the end of the experiment, in contrast to around 95% release from the free SeNPs. Notably, within the first 22 hours, 75% of free SeNPs diffused out, whereas liposomal SeNPs showed a restrained release of about 24%. The kinetic profile of the liposome-based formulation demonstrates a biphasic pattern: an initial burst release phase during the early hours (0–22 h), followed by a sustained, gradual release extending through the remainder of the study period. This sustained release behavior suggests that liposomal encapsulation effectively moderates drug diffusion, potentially enhancing the therapeutic window and reducing off-target effects.
In Vitro Cytotoxicity Evaluation
The cytotoxic effects of both free and liposome- encapsulated selenium nanoparticles (SeNPs) were evaluated on the HSC-3 oral squamous carcinoma cell line using the MTT assay. As shown in Figure 2, liposomal SeNPs exhibited significantly higher cytotoxicity compared to free SeNPs. After 24 hours of treatment, the liposome-loaded formulation reduced cell viability to 28 ± 4%, whereas the free SeNPs maintained a higher viability of 62 ± 3%. This difference was statistically significant (p < 0.001), indicating that liposomal encapsulation enhances the antiproliferative effect of SeNPs.
Figure 2. In vitro Cytotoxicity of Free SeNPs, Liposome Loaded SeNPs, and free liposome on HSC-3 cell line. n=3, *** p <0.001 .
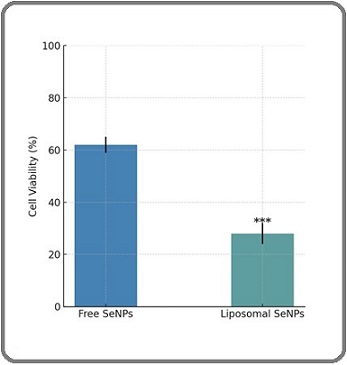
These findings suggest that the liposomal formulation facilitates improved cellular uptake or prolonged intracellular retention, thereby potentiating the therapeutic impact against oral cancer cells.
Discussion
Oral squamous cell carcinoma (OSCC) remains one of the most prevalent and aggressive malignancies of the head and neck region, accounting for over 90% of oral cancers [59, 60]. Despite advances in surgery, chemotherapy, and radiotherapy, OSCC continues to pose significant therapeutic challenges due to late diagnosis, local recurrence, metastasis, and treatment-related toxicity [61-63]. Conventional chemotherapeutic agents such as cisplatin, 5-fluorouracil, and paclitaxel, though widely used, often suffer from limited selectivity, poor bioavailability, and severe systemic side effects that compromise patient outcomes and quality of life [60, 64, 65]. In recent years, nanotechnology-based drug delivery systems particularly liposomal carriers have gained considerable attention for their ability to overcome many of these limitations [66]. Liposomes are biocompatible, phospholipid-based vesicles capable of encapsulating both hydrophilic and hydrophobic agents. Their ability to prolong circulation time, enhance tumor-specific accumulation via the enhanced permeability and retention (EPR) effect, and reduce nonspecific toxicity makes them ideal candidates for oral cancer therapeutics. Several studies have demonstrated that liposomal formulations of conventional anticancer agents can significantly enhance antitumor efficacy while minimizing damage to healthy tissues [67-69]. In this context, the present study introduces a novel formulation: green-synthesized selenium nanoparticles (SeNPs) encapsulated within liposomes, aimed at improving OSCC treatment. The findings validate the hypothesis that encapsulating SeNPs in phospholipid liposomes not only improves their physicochemical stability but also significantly boosts their therapeutic performance [66, 70]. The green synthesis approach using Trifolium cherleri extract provided an eco-friendly and cost-effective route to produce biocompatible SeNPs [71]. The observed color change from pale yellow to orange-red upon mixing the extract with sodium selenite confirms successful bioreduction, consistent with previous phytogenic synthesis studies [72]. This method offers an alternative to conventional chemical synthesis, reducing the risk of toxic byproducts and supporting scalability for biomedical applications [73]. The thin-film hydration method yielded liposomal SeNPs with a uniform particle size of approximately 235 nm and a narrow PDI (~0.15), indicative of monodisperse nanoscale vesicles. A zeta potential of –28.6 mV suggests sufficient electrostatic repulsion to prevent aggregation, in line with colloidal stability requirements for drug delivery systems [74, 75]. A critical advantage of the liposomal formulation was the significantly slower release kinetics observed in the in vitro drug release study. While ~95% of free SeNPs diffused within 62 hours, the encapsulated formulation released only ~35% of the payload in the same duration. This biphasic release initial burst followed by sustained release demonstrates the controlled-release capabilities of the liposomal bilayer, potentially reducing systemic toxicity and maintaining therapeutic levels over time [76, 77]. Functionally, the cytotoxicity assessment confirmed that liposomal SeNPs exhibited markedly enhanced anticancer activity against the HSC-3 OSCC cell line, reducing cell viability to 28% after 24 hours. This is significantly superior to the 62% viability seen with free SeNPs (p < 0.001). Such improved potency may be attributed to enhanced cellular uptake via endocytosis of the liposomes, as well as prolonged intracellular retention compared to non-encapsulated nanoparticles. Moreover, the lack of significant cytotoxicity in unloaded liposomes reinforces the biocompatibility of the delivery vehicle [78-80]. These results are in strong agreement with prior reports demonstrating the utility of liposomal carriers for elemental selenium delivery. Similar studies using folic acid-modified liposomes or polysaccharide-coated SeNPs have also shown enhanced tumor selectivity and reduced off-target effects, underscoring the potential of surface-engineered systems in precision oncology [81]. Despite these promising in vitro outcomes, further research is necessary. The mechanism of cellular uptake, possible endosomal escape, and the role of phytoconstituents in mediating cytotoxicity remain unexplored. Moreover, in vivo validation in animal models is crucial to assess pharmacokinetics, biodistribution, tumor-targeting efficiency, and long-term toxicity. These data are essential prerequisites for clinical translation [82].
In summary, this study provides a compelling proof-of-concept that phytogenically synthesized SeNPs, when encapsulated in liposomes, exhibit enhanced colloidal stability, controlled release, and superior antitumor efficacy in vitro. These findings contribute to the growing body of evidence supporting nanotechnology- based drug delivery as a potent strategy for oral cancer treatment and lay the groundwork for future in vivo and clinical investigations.
Statements and Declarations
Funding
The authors have no relevant financial or non-financial interests to disclose.
Conflict of interests
The authors have no competing interests to declare that are relevant to the content of this article.
Ethics approval
This study did not involve any original data collection or human subjects, and therefore, ethical approval was not required.
Consent
All authors have provided consent for publication.
References
- Regulation of Dendritic Cell Functions by Vitamins as Promising Therapeutic Strategy for Immune System Disorders Ghahramanipour Z, Alipour S, Masoumi J, Rostamlou A, Hatami-Sadr A, Heris JA , Naseri B, et al . Advanced Biology.2023;7(12). CrossRef
- Combination of B7H6-siRNA and temozolomide synergistically reduces stemness and migration properties of glioblastoma cancer cells Allahyarzadeh Khiabani N, Amin Doustvandi M, Mohammadnejad F, Salmani Hassan Kohal E, Boushehri N, Jafarlou M, Baradaran B. Experimental Cell Research.2023;429(1). CrossRef
- The Combination of PD-L1 and CTLA-4 Suppression Significantly Decreased the Expression Levels of Cancer Stem Cell Factors in the Pancreatic Cancer Cell Line Alizadeh N, Kazemi T, Hemmat N, Jafarlou M, Baradaran B. ImmunoAnalysis, 3(1), 6-6..2024. CrossRef
- Characteristics and Cytotoxic Effects of Nano-Liposomal Paclitaxel on Gastric Cancer Cells Abedi Cham Heidari Z, Ghanbarikondori P, Mortazavi Mamaghani E, Hheidari A, Saberian E, Mozaffari E, Alizadeh M, Allahyartorkaman M. Asian Pacific journal of cancer prevention: APJCP.2023;24(9). CrossRef
- Dendrite neural network scheme for estimating output power and efficiency for a class of solar free-piston Stirling engine Lashaki RA, Raeisi Z, Makki M, Zare S. International Journal of Modelling and Simulation.;0(0). CrossRef
- Comprehensive GIS-driven evaluation of drought severity and duration: comparative assessment of parametric and non-parametric SPI methodologies Mirdarsoltany A, Dariane AB , Borhan MI . Theoretical and Applied Climatology.2025. CrossRef
- Linking Land Use Change and Hydrological Responses: The Role of Agriculture in the Decline of Urmia Lake Mirdarsoltany A, Dariane AB , Ghasemi M, Farhoodi S, Asadi R, Moghaddam A. Hydrology, 11(12), 209..2025. CrossRef
- Application of Scaffold-Based Drug Delivery in Oral Cancer Treatment: A Novel Approach Saberian E, Jenča A, Petrášová A, Zare-Zardini H, Ebrahimifar M. Pharmaceutics.2024;16(6). CrossRef
- Scaffold Application for Bone Regeneration with Stem Cells in Dentistry: Literature Review Saberian E, Jenča A, Zafari Y, Jenča A, Petrášová A, Zare-Zardini H, Jenčová J. Cells.2024;13(12). CrossRef
- Comparative Analysis of Osteoblastic Responses to Titanium and Alumina-Toughened Zirconia Implants: An In Vitro Study Saberian Elham , et al . ResearchGate.2025. CrossRef
- From defense to offense: antimicrobial peptides as promising therapeutics for cancer Zare-Zardini H, Saberian E, Jenča A, Ghanipour-Meybodi R, Jenča A, Petrášová A, Jenčová J. Frontiers in Oncology.2024;14. CrossRef
- Dental Pulp Stem Cells in Pulp Regeneration Saberian E, et al . SunText Review of Medical & Clinical Research.2021;2(3). CrossRef
- Seismic Analysis of Rectangular Alluvial Valleys Subjected to Incident SV Waves By Using the Spectral Finite Element Method Najafizadeh J, Kamalian M, Jafari MK , Khaji N. International Journal of Civil Engineering.2014;12(3).
- Seismic Nonlinear Behaviour Of Rectangular Alluvial Valleys Subjected To Vertically Propagating Incident Sv Waves Using The Spectral Finite Element Method Najafizadeh, Jafar , Mohsen Kamalian , Mohammad Kazem Jafari , Peyman Aminpour . In Proceedings Of The 7th International Conference On Seismology & Earthquake Engineering.2015;:18-21.
- Machine learning for earthquake engineering analysis: Comparing regression models to predict peak ground acceleration Pakniat, Shima , Jafar Najafizadeh , Monavvareh Kadkhodaavval . World Journal of Advanced Research and Reviews, 2025, 26(02), 856-867. Article DOI: https://doi.org/10.30574/wjarr.2025.26.2.1714.2025.
- Study of solute-solvent interactions using volumetric properties for the ternary {L-Serine +H2O+NaBr, KBr, LiBr} solutions at different temperatures and ambient pressure Ghasemi H, Rafiee HR . Chemical Data Collections.2020;29. CrossRef
- The effect of tart cherry juice (TCJ) supplementation on exercise-induced muscle damage (EIMD) in an athletic population Dehghani E, Beba M, Danandeh K, Memari A, Ershadmanesh MJ , Rasoulian P, Danandeh A, Djafarian K. Annals of Medicine and Surgery (2012).2025;87(2). CrossRef
- A newly generated seismic ground response analysis software package - SeisGRASP - by International Institute of Earthquake Engineering and Seismology Jalili J, J, Moosavi M M, Pakniat S. Iran J Sci Technol Trans Civ Eng.2024;48:1467-1482.
- Effect of Seismic Site Response on Damage Distribution in Sarpol-e Zahab City Caused by 12 November 2017 Mw 7.3 Strong Ground Motion: Fooladi area Pakniat S, Moosavi M, Jalili J. Journal of Seismology and Earthquake Engineering.2021;23(3). CrossRef
- Seismic Response of 2D Triangular-Shaped Alluvial Valleys to Vertically Propagating Incident SV Waves Aminpour P, Najafizadeh J, Kamalian M, Jafari MK . Journal of Seismology and Earthquake Engineering.2015;17(2).
- Dual silencing of tumor-intrinsic VISTA and CTLA-4 stimulates T-cell mediated immune responses and inhibits MCF7 breast cancer development Hosseinkhani N, Hemmat N, Baghbani E, Baghbanzadeh A, Kazemi T, Mokhtarzadeh A, Jafarlou M, Amin Doustvandi M, Baradaran B. Gene.2024;896. CrossRef
- Berberis Vulgaris Fruit Crude Extract As A Novel Anti-Leukaemic Agent Saedi T. A., Ghafourian S., Jafarlou M., Sabariah M. N., Ismail P., Eusni R. M. T., Othman F.. Journal of Biological Regulators and Homeostatic Agents.2015;29(2).
- Optimized classification of dental implants using convolutional neural networks and pre-trained models with preprocessed data Lashaki RA , Raeisi Z, Razavi N, Goodarzi M, Najafzadeh H. BMC Oral Health.2025;25(1). CrossRef
- Multi-Step Ahead Water Level Forecasting Using Deep Neural Networks Sharafkhani F, Corns S, Holmes R. Water.2024;16(21). CrossRef
- Introducing a novel temperature measurement to analyze the effect of hybrid cooling methods on improving solar panel performance: An experimental approach Aali M, Mansouri M, Raeisi Z, Lashaki RA , Tavakoli S. Applied Thermal Engineering.2025;268. CrossRef
- Chronic effects of tobacco smoking on electrical brain activity: A systematic review on electroencephalography studies Taebi M, Taghavizanjani F, Parsaei M, Ershadmanesh M, Beikmarzehei A, Gorjestani O, Rezaei Z, Hasanzadeh A, Moghaddam HS . Behavioural Brain Research.2025;484. CrossRef
- The emerging role of noncoding RNAs in systemic lupus erythematosus: new insights into the master regulators of disease pathogenesis Afrashteh Nour M, Ghorbaninezhad F, Asadzadeh Z, Baghbanzadeh A, Hassanian H, Leone P, Jafarlou M, Alizadeh N, Racanelli V, Baradaran B. Therapeutic Advances in Chronic Disease.2023;14. CrossRef
- Unveiling the menace: a thorough review of potential pandemic fungal disease Jafarlou M. Frontiers in Fungal Biology.2024;5. CrossRef
- Studying the Characteristics of Curcumin-Loaded Liposomal Nanoparticles Afyouni I, Ghanbarikondori Pa, Pour NS , Hashemian PM , Jalali F, Sedighi A, Allahyartorkaman M. Asian Pacific Journal of Cancer Biology.2024;9(2). CrossRef
- First report of isolation and identification of Brevundimonase (Pseudomonas) diminuta from collected nasopharyngeal specimens in suspected patients to pertussis Mirzaei B, Torkaman MRA , Babaei R, Shahcheraghi* F. African Journal of Microbiology Research.2014;8(11). CrossRef
- Common Dietary Patterns Among Female Employees Participating in the Persian Cohort Study (Mashhad) and Their Relationship With Metabolic Syndrome Dastenaie FD , Javaheri FSH , Masoumvand M, Manesh MAN , Moghadam MA , Khosravi M, Esfehani AJ . Health Science Reports.2025;8(1). CrossRef
- Comparison of the Effect of Intermittent Fasting with Mediterranean Diet on Glycemic, Lipid, and Anthropometric Indices in Type 2 Diabetes: A Review of Randomized Controlled Trials Dehghani S, Karimi P, Tarei NM , Masoumvand M, Manesh MAN , Ramezani E, Askari VR . Current Hypertension Reviews.2025. CrossRef
- Effect of Acoustic Cavitation on Mouse Spermatogonial Stem Cells: Colonization and Viability Moghaddam ZH , Mokhtari-Dizaji M, Movahedin M. Journal of Ultrasound in Medicine: Official Journal of the American Institute of Ultrasound in Medicine.2021;40(5). CrossRef
- Enhanced LSTM by attention mechanism for early detection of Parkinson’s disease through voice signals. In: 2023 2nd int. eng. conf. electr. energy, artif. intell Mohammadigilani A, Attar H, Chimeh HE , Karami M. 2023;:1–5. CrossRef
- pH-Responsive Microneedle Actuator Array for Precise Wound Healing: Design, Actuation, Light Filtering, and Evaluation. In 2024 IEEE 17th Dallas Circuits and Systems Conference (DCAS) Pour MR , Tan JY , Saha R, Kim A , Kim J . IEEE.2024;:1-4. CrossRef
- Integrative Cancer Care: Leveraging Nutrition and Positive Psychology for Optimal Outcomes Maryam Arabmoorchegan , Mahsa Abbasi , Mahya Asadalizadeh , Forough Motavaf . APJCN.2025. CrossRef
- Regulatory Effects of Apatinib in Combination with Piperine on MDM-2 Gene Expression, Glutathione Peroxidase Activity and Nitric Oxide level as Mechanisms of Cytotoxicity in Colorectal Cancer Cells Mohammadian M, Rostamzadeh Khameneh Z, Emamgholizadeh Minaei S, Ebrahimifar M, Esgandari K. Advanced Pharmaceutical Bulletin.2022;12(2). CrossRef
- Carboplatin and epigallocatechin-3-gallate synergistically induce cytotoxic effects in esophageal cancer cells Taghvaei F, Rastin SJ , Milani AT , Khameneh ZR , Hamini F, Rasouli MA , Asghari K, Rekabi Shishavan AM , Ebrahimifar M, Rashidi S. Research in Pharmaceutical Sciences.2021;16(3). CrossRef
- Environmental Determinants of Oral Cancer Development: An Overview Maddahi M, Ghanbarikondori P, Amiri F, Abdi N, Jahromi AM , Pour NS , Allahyartorkaman M, Moazzam F. Asian Pacific Journal of Environment and Cancer.2024;7(1). CrossRef
- Enhancing Cisplatin Delivery via Liposomal Nanoparticles for Oral Cancer Treatment Ghanbarikondori P, Aliakbari RBS , Saberian E, Jenča A, Petrášová A, Jenčová J, Khayavi AA . Indian journal of clinical biochemistry: IJCB.2025;40(2). CrossRef
- Utilizing Niosome Nanoparticles for the Combined Treatment of Curcumin and Cisplatin in Oral Cancer Rezaei, Fatemeh , Tara Fesharakinia , Soheil Balsini Gavanaroudi , Maryam Rezaeianjam , Mahyar Khanlari Goodarzi , Maasoume Abdollahi , Kiomars Akaberi . Asian Pacific Journal of Cancer Biology.2024;9(4):569-577. CrossRef
- Folic Acid-Conjugated Nanoniosomes: An Effective Carrier for Targeted Bleomycin Delivery in Oral Cancer Salehan F, Mohammadi Y, Shieh M, Askarizadeh M, Rahmani S, Alishahi F, Hheidari A. Asian Pacific Journal of Cancer Biology.2025;10(1):63-70. CrossRef
- Enhanced Anticancer Potential of Curcumin-Loaded Liposomal Nanoparticles in Oral Cancer Treatment Moravedeh R, Samadnezhad NZ , Asadalizadeh M, Abbasi M, Nadaki A. Asian Pacific Journal of Cancer Biology.2025;10(2). CrossRef
- A Review of Poly Butyl Cyanoacrylate Nanoparticles as a Cancer Drug Delivery and Targeting Semyari S, Azizi S, Kundu D, Boroumandmoghaddam A, Moniri M, Ebrahimifar M, Toofani Milani A. Journal of Nanostructures.2021;11(4). CrossRef
- Investigation the characteristics of carboplatin loaded onto pegylated liposomal nanoparticles on the rat Glioma cell line C6 Izadi M, Shahemabadi HE , Kanaani L, Sardari KA , Ebrahimifar M, Safdari F, Moradi-Sardareh H. Adv Biores.2016;7:113-118.
- Bleomycin-loaded folic acid-conjugated nanoliposomes: a novel formulation for targeted treatment of oral cancer Saberian E, Jenčová J, Jenča A, Jenča A, Salehipoor F, Zare-Zardini H, Petrášová A, et al . Frontiers in Bioengineering and Biotechnology.2025;13. CrossRef
- Targeting Nanog expression increased Cisplatin chemosensitivity and inhibited cell migration in Gastric cancer cells Vasefifar P, Najafi S, Motafakkerazad R, Amini M, Safaei S, Najafzadeh B, Alemohammad H, Jafarlou M, Baradaran B. Experimental Cell Research.2023;429(2). CrossRef
- Nanoliposomes Meet Folic Acid: A Precision Delivery System for Bleomycin in Cancer Treatment Shakiba D, Shabestari AM , Mokhtari T, Goodarzi MK , Saeed S, Zinatbakhsh Z, Akaberi K, Allahyartorkaman M. Asian Pacific Journal of Cancer Biology.2024;9(4). CrossRef
- Enhanced Anticancer Efficacy of Selenium Nanoparticles Encapsulated in Niosomes: A Novel Therapeutic Strategy Amiri F, Alishahi F, Mohammadifar G, Izadidehkordi S, Charmduzi F, Dialameh F, Khiyavi AA . Indian Journal of Clinical Biochemistry.2025;:1-6. CrossRef
- Combined sonodynamic therapy and X-ray radiation with methylene blue and gold nanoparticles coated with apigenin: Impact on MCF7 cell viability Neshastehriz A., Hormozi-Moghaddam Z., Amini S. M., Taheri S. M., Abedi Kichi Z.. International Journal of Radiation Research.2024;22(2). CrossRef
- Selenium nanoparticles fabricated in Undaria pinnatifida polysaccharide solutions induce mitochondria-mediated apoptosis in A375 human melanoma cells Chen T, Wong Y, Zheng W, Bai Y, Huang L. Colloids and Surfaces. B, Biointerfaces.2008;67(1). CrossRef
- Anti-neoplastic selenium nanoparticles from Idiomarina sp. PR58-8 Srivastava P, Kowshik M. Enzyme and Microbial Technology.2016;95. CrossRef
- Nanomedicine Assembled by Coordinated Selenium-Platinum Complexes Can Selectively Induce Cytotoxicity in Cancer Cells by Targeting the Glutathione Antioxidant Defense System Li F, Li T, Han X, Zhuang H, Nie G, Xu H. ACS biomaterials science & engineering.2018;4(6). CrossRef
- Betacyanins functionalized selenium nanoparticles inhibit HepG2 cells growth via mitochondria-mediated pathway Tang X, Yu S, Guo X, Li H, Chen M, Zhang T, Lei C, Zhao Z, Meng H. Journal of Functional Foods.2021;78. CrossRef
- Facile synthesis of highly uniform selenium nanoparticles using glucose as the reductant and surface decorator to induce cancer cell apoptosis Nie T, Wu H, Wong K, Chen T. Journal of materials chemistry. B.2016;4(13):2351-2358. CrossRef
- Lentinan-functionalized selenium nanoparticles induce apoptosis and cell cycle arrest in human colon carcinoma HCT-116 cells Gao X, Yao Y, Chen X, Lin X, Yang X, Ho C, Li B, Chen Z. Frontiers in Nutrition.2022;9. CrossRef
- Chemopreventive mechanism of action by oxidative stress and toxicity induced surface decorated selenium nanoparticles Menon S, Shanmugam VK. Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements (GMS).2020;62. CrossRef
- Potentiation of in Vivo Anticancer Efficacy of Selenium Nanoparticles by Mushroom Polysaccharides Surface Decoration Zeng D, Zhao J, Luk K, Cheung S, Wong K, Chen T. Journal of Agricultural and Food Chemistry.2019;67(10). CrossRef
- Manajemen multidisiplin Oral Squamous Cell Carcinoma (OSCC): laporan kasus. Riskayanti NPD , Riyanto D, Winias S. Intisari Sains Medis.2021.
- The role of non-coding RNAs in drug resistance of oral squamous cell carcinoma and therapeutic potential Meng X, Lou Q, Yang W, Wang Y, Chen R, Wang L, Xu T, Zhang L. Cancer Communications (London, England).2021;41(10). CrossRef
- Complete remission in very advanced oral cancer by docetaxel, cisplatin, 5-fluorouracil based induction chemotherapy followed by concurrent chemoradiation Chiang T, Ho C, Lin C, Chen Y. Journal of Dental Sciences.2018;13(1). CrossRef
- The Molecular Basis and Therapeutic Aspects of Cisplatin Resistance in Oral Squamous Cell Carcinoma Cheng Y, Li S, Gao L, Zhi K, Ren W. Frontiers in Oncology.2021;11. CrossRef
- Neoadjuvant concurrent radiochemotherapy followed by surgery in advanced oral squamous cell carcinoma (OSCC): a retrospective analysis of 207 patients Freier K, Engel M, Lindel K, Flechtenmacher C, Mühling J, Hassfeld S, Hofele C. Oral Oncology.2008;44(2). CrossRef
- Phase II study of tolerance and efficacy of hyperfractionated radiotherapy and 5-fluorouracil, cisplatin, and paclitaxel (Taxol) in stage III and IV inoperable and/or unresectable head-and-neck squamous cell carcinoma: A-2 protocol Abitbol A, Abdel-Wahab M, Lewin A, Troner M, Rodrigues M, Hamilton-Nelson KL , Markoe A. International Journal of Radiation Oncology, Biology, Physics.2002;53(4). CrossRef
- Weekly Paclitaxel, Carboplatin, and Cetuximab as First-Line Treatment of Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma for Patients Ineligible to Cisplatin-Based Chemotherapy: A Retrospective Monocentric Study in 60 Patients Carinato H, Burgy M, Ferry R, Fischbach C, Kalish M, Guihard S, Brahimi Y, et al . Frontiers in Oncology.2021;11. CrossRef
- Exploring the therapeutic potential of lipid-based nanoparticles in the management of oral squamous cell carcinoma Chaudhary AA , Fareed M, Khan S, Alneghery LM , Aslam M, Alex Ar, Rizwanullah M. Exploration of Targeted Anti-Tumor Therapy.2024;5(6). CrossRef
- Controlled Drug Delivery Systems for Oral Cancer Treatment-Current Status and Future Perspectives Ketabat F, Pundir M, Mohabatpour F, Lobanova L, Koutsopoulos S, Hadjiiski L, Chen X, et al . Pharmaceutics.2019;11(7). CrossRef
- Mucoadhesive Nanocarriers as a Promising Strategy to Enhance Intracellular Delivery against Oral Cavity Carcinoma Pandey M, Choudhury H, Ying JNS , Ling JFS , Ting J, Ting JSS , Zhia Hwen IK , et al . Pharmaceutics.2022;14(4). CrossRef
- A novel intra-tumoral drug delivery carrier for treatment of oral squamous cell carcinoma Elsaady SA , Aboushelib MN , Al-Wakeel E, Badawi MF . Scientific Reports.2023;13(1). CrossRef
- A Detailed Review on the Green Synthesis of Selenium Nanoparticles Using Plant Extracts and Their Anticancer Applications Tarhan T. ChemistrySelect.2024;9(41). CrossRef
- Green Synthesis of Selenium Nanoparticles: Characterization and Therapeutic Applications in Microbial and Cancer Treatments Yasodha S, Vickram A.s , Rajeshkumar S. International Research Journal of Multidisciplinary Technovation.2024. CrossRef
- Synthesis, characterisation, and in vitro biocompatibility studies of selenium nanoparticles synthesized using Hybanthus enneaspermus plant extract Veeraraghavan VP , Chandran ABS , Manivannan HP , et al. . Texila Int J Public Health.2023.
- Biosynthesis of selenium nanoparticles using plant extracts Pyrzyńska K, Sentkowska A. J Nanostruct Chem.2024. CrossRef
- Exploring the liposomal encapsulation and enhanced cytotoxicity of selenium nanoparticles against lung cancer cells Chirakara D, Lotlikar S, Nannan M, Dunna NR , Venkatabalasubramanian S. Next Nanotechnology.2025;7. CrossRef
- Characteristics of liposome used for drug delivery system Khan W. Journal of Biomedical and Pharmaceutical Research.2016;5(5). CrossRef
- Design and Characterization of a Cancer-Targeted Drug Co-Delivery System Composed of Liposomes and Selenium Nanoparticles Xuan G, Zhang M, Chen Y, Huang S, Lee I. Journal of Nanoscience and Nanotechnology.2020;20(9). CrossRef
- A comprehensive mathematical model of drug release kinetics from nano-liposomes, derived from optimization studies of cationic PEGylated liposomal doxorubicin formulations for drug-gene delivery Haghiralsadat F, Amoabediny G, Helder MN , Naderinezhad S, Sheikhha MH , Forouzanfar T, Zandieh-Doulabi B. Artificial Cells, Nanomedicine, and Biotechnology.2018;46(1). CrossRef
- Selective Cytotoxicities of Red-Allotrope Selenium Nanoparticles and Polyethylene Glycol Towards Head and Neck Squamous Cell Carcinoma in Comparison to Human Dermal Fibroblasts Hassan C, Webster TJ . MRS Advances.2016;1(56). CrossRef
- GE11 peptide conjugated selenium nanoparticles for EGFR targeted oridonin delivery to achieve enhanced anticancer efficacy by inhibiting EGFR-mediated PI3K/AKT and Ras/Raf/MEK/ERK pathways Pi J, Jiang J, Cai H, Yang F, Jin H, Yang P, Cai J, Chen ZW . Drug Delivery.2017;24(1). CrossRef
- Doxorubicin-loaded functionalized selenium nanoparticles for enhanced antitumor efficacy in cervical carcinoma therapy Xia Y, Xiao M, Zhao M, Xu T, Guo M, Wang C, Li Y, Zhu B, Liu H. Materials Science & Engineering. C, Materials for Biological Applications.2020;106. CrossRef
- The anticancer effect of magnetic selenium-based nanocomposites on tongue carcinoma stem cells Mohamed LMK , Farag DBE , Beherei H, AbuBakr N. BioNanoScience.2021;12:1-12.
- Evaluation of selenium nanoparticles and doxorubicin effect against hepatocellular carcinoma rat model cytogenetic toxicity and DNA damage Abd El-Moneim OM , Abd El-Rahim AH , Hafiz NA . Toxicology Reports.2018;5. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details