A Single-Institutional Prospective Study Comparing Clinical Outcome and Toxicities of 1-Week Versus 3-Week Adjuvant Radiotherapy in Post-Mastectomy Breast Carcinoma: 3-Year Follow-Up
Download
Abstract
Objective: Recent data showed 1-week schedule as non-inferior to the 3-week schedule of adjuvant radiotherapy in breast carcinoma in terms of local control and normal tissue toxicity. In governmental set-ups for mostly poor patients, it is highly desirable to validate this data and apply in practice. This study was done to compare recurrence-free survival, and acute and late toxicities of these two schedules.
Methods: In this prospective, observational, single-institutional study, post-mastectomy breast carcinoma patients (pT3N0M0; or early disease with positive margin) were 1:1 divided in two groups: Arm A received adjuvant chest wall radiation 26Gy in 5 fractions over 1 week, and Arm B received 40Gy in 15 fractions over 3 weeks. Patients with both arms completed adjuvant therapy, and they were followed up 3-monthly for 1 year, then 6-monthly. Acute and late toxicities were assessed using Common Terminology Criteria for Adverse Events (CTCAE) v5.0. Statistical analysis was done using SPSS v30.0.
Results: Between May 2020 and October 2021, total 43 patients (Arm A – 20, Arm B – 23) were treated by 3D Conformal Radiation Therapy in VARIAN TrueBeam, and were followed-up till December 2024. Over a median follow-up of 42 months, 1 patient in Arm A (5%) and 2 in Arm B (8.7%) had chest wall recurrence (Odds ratio 0.5526, p-value 0.6392). Mean RFS for Arm A and Arm B were 51.150 months (95% CI 49.526 – 52.774) and 50.633 months (95% CI 48.805 – 52.460) respectively (p-value 0.691). No significant difference in acute skin toxicity was observed between two arms. At 3 years of follow-up, grade 1 chronic cough was seen in 5% of Arm A and 8.7% of Arm B (p-value 0.6392), grade 1 pulmonary fibrosis in 5% of Arm A and 4.35% of Arm B (p-value 0.9194), and grade 1 cardiac chest pain in 10% of Arm A and 4.35% of Arm B (p-value 0.4799) without any serious cardiovascular event.
Conclusion: No statistically significant difference was observed between the 1-week (26Gy in 5 fractions) schedule and the standard 3-weekly regimen of adjuvant radiotherapy in post-mastectomy node-negative breast cancer patients in terms of local control, and acute and late skin, pulmonary and cardiac toxicities.
Introduction
Radiation therapy (RT) is an essential part of the multimodality management of carcinoma breast in terms of local tumour control [1, 2]. Post-Mastectomy Radiation Therapy (PMRT) has been shown to significantly reduce the risk of 10-year loco-regional and overall recurrence, as well as improve the 20-year breast cancer mortality rates by 8% in node-positive patients [3, 4]. Historically, the most frequently used schedule of adjuvant radiation therapy worldwide was a total dose of 50Gy delivered in 25 fractions of 2Gy per day, 5 days a week during 5 weeks, with or without a subsequent boost [5]. Some studies have shown that a moderately hypo-fractionated treatment consisting of 15–16 fractions over 3 weeks is associated with comparable efficacy and toxicity to the conventional schedule, but in a shorter time period [6, 7]. Breast being a late reacting tissue, this hypo-fractionation regimen (40Gy in 15 fractions, 2.67Gy per fraction each day, 5 days a week, over 3 weeks) has benefits in terms of radiobiology as well as logistics , thus quickly becoming the international standard of care [7].
Recently, FAST and FAST-Forward trials attempted even more extreme hypo-fractionated radiation schedules [8, 9]. In FAST, 28.5Gy or 30Gy in 5 fractions showed similar cosmetic outcomes to the conventional 2Gy per-fraction schedule for low-risk breast cancer [8]. FAST-Forward trial investigated ipsilateral breast tumour relapse (IBTR) after chest wall/whole breast irradiation with 26Gy and 27Gy in 5 fractions over a week demonstrating non-inferiority of the 26Gy/5# schedule to the standard 40Gy/15# schedule in terms of local control and normal tissue toxicity at 5 years of follow-up [9]. The results from its nodal sub-study indicate that at 3 years follow-up there is no early indication that outcomes relating to arm or shoulder adverse effects are different for 26 Gy compared with the standard regimen [10].
Since the results of these trials have been published, ultra-hypo-fractionated 1-week schedules have gained support from various studies all over the world, showing comparable tumour control rates and toxicity to the current standard. 26Gy in 5 fractions over a week is emerging as a new standard for certain breast irradiation scenarios, though data supporting nodal or boost irradiation are limited [11, 12]. This study is very relevant in our setting where many patients with breast cancer present to us with limited resources that preclude the use of a long schedule of radiation. In a third-world country like India, a shorter course of radiation with same efficacy is highly desirable in terms of patient compliance, logistics and reducing the cost of the treatment and the loss of their daily wages. During the first and second waves of the COVID-19 pandemic, the 1-week schedule gained profound significance as per the need of the hour. Based on this, we conducted our study in post-mastectomy early-stage breast carcinoma patients comparing this ultra-hypo-fractionated schedule (26Gy/5 fractions) to routinely practised moderate hypofractionation schedule (40Gy/15fractions) to better understand its effects in Indian population, and to determine whether its safe routine practice is possible in near-future.
Materials and Methods
Aim of the study
To compare recurrence-free survival (RFS), and the acute and late (skin, pulmonary and cardiac) toxicities between 1-week and 3-week schedule.
Design of the study
Prospective, longitudinal study.
Setting of the study
Department of Radiation Oncology, Medical College and Hospital, Kolkata, West Bengal, India.
A. Patient Selection
Between May 2020 and October 2021, all the patients with histologically proven invasive breast carcinoma attending the Radiation Oncology department of Medical College Kolkata after upfront mastectomy and adequate axillary clearance were planned for adjuvant treatment. Among them, 43 patients fulfilled the inclusion criteria (TNM staging pT3 N0 M0 with or without R0 resection, or any lower stage with positive margin, with all of the following - age 25-60 years, female breast cancer, Karnofsky Performance Status > 70, normal baseline pulmonary function test and left ventricular ejection fraction) and exclusion criteria (metastatic breast cancer, pT4a/T4b disease, positive nodal status, age <25 years and >60 years, post- Breast Conservation Surgery patients, pulmonary and/or cardiac comorbidities, previous chest wall irradiation, previous malignancy, arm oedema, history of collagen vascular diseases, COVID positive). These patients were 1:1 divided into two groups – 20 patients in Arm A to receive chest wall irradiation 26Gy in 5 fractions, 5 days a week, over 1 week, and 23 patients in Arm B to receive chest wall irradiation 40Gy in 15 fractions, 5 days a week, over 3 weeks, i.e., open-label randomization was done in the allocation of the patients.
After taking clearance from the Institutional Ethics Committee, the prospective longitudinal study started at Medical College Kolkata. Informed consent was taken from each of the patients.
B. Simulation, contouring and planning
Our institutional Philips Brilliance 16-slice CT scan machine was used to acquire simulation scans of 3mm slice width. Patients were in supine position lying on zero-degree cushion, both arms raised above head using arm-rest, head straight, immobilised using a 4-clamp thermoplastic thoraco-abdominal mask. Metallic wire was used to mark the mastectomy scar on skin. Free breathing technique was used. Contouring of the target volume and the organs-at-risk (OARs) was done according to Radiation Therapy Oncology Group (RTOG) guidelines at Varian SomaVision workstation [13]. OARs included heart, ipsilateral lung, combined lungs, and contralateral breast. A 7mm margin was given for Planning Target Volume (PTV) around the Clinical Target Volume (CTV), and the PTV was cropped from the skin by 3mm as per institutional protocol.
Three-dimensional conformal radiation therapy (3DCRT) plan for each patient was done by our medical physicists using Varian Eclipse software, and dose profile to chest wall and OARs were checked during plan evaluation. Dose constraints to the OARs in Arm A were set to V7Gy < 5% (Heart), V8Gy < 15% (ipsilateral lung), and for Arm B, V10Gy < 5% (Heart), V12Gy < 15% (ipsilateral lung). For left-sided breast patients, the heart constraints were set to V7Gy < 7.5% and V10Gy < 7.5% for Arm A and B, respectively. V20Gy < 20% (combined lungs) and Dmax < 1.6Gy (contralateral breast) were used for both arms. After the clinicians’ approval of the plans, the patients were treated in VARIAN TrueBeam linear accelerator (serial number – 3279). Cone beam CT scan was taken before first treatment to match with the planning CT, and then for the first 3 days, and then weekly for Arm B.
C. Follow-up parameters
Before starting treatment, baseline 2D echocardiography and pulmonary function tests were done along with complete blood count (CBC), liver and renal function tests (LFT, KFT). During the radiation, blood counts were checked weekly for Arm B, and once after completion in Arm A. After radiation completion, the adjuvant systemic therapy was continued for the patients with or without hormone and anti-HER therapy according to their ER-PR-HER2neu status.
Patients were checked just after the completion of the radiation treatment, and then followed up monthly for 1st 3 months, then 3-monthly for the first year, and 6-monthly for the second and third year. Clinical local examination, high-resolution ultrasonography of chest wall, opposite breast and bilateral axilla, digital chest X-ray, routine blood tests (CBC, KFT, LFT), pulmonary function tests and 2D echocardiography were done in each follow-up. High-resolution computed tomography (HRCT) scans were done only for patients with chronic dry cough, to rule out pulmonary fibrosis. Acute and late toxicities were assessed using Common Terminology Criteria for Adverse Evens (CTCAE) V5.0 [14].
D. Statistical analysis
Continuous variables were tested with Mann-Whitney test[15] as it is a commonly used nonparametric test for comparing two groups of independent samples, while categorical variables were tested by Fisher’s exact test[16] due to its validity in analysing small sample of categorical variables and providing an exact p-value. Odds ratios were calculated between the two arms with respect to the acute and late toxicities. Kaplan-Meier survival analysis was done, and the survival outcome between the two arms were compared using log-rank test. A p-value <0.05 was considered significant. All the statistical analysis was done using Statistical Package for the Social Sciences (SPSS) software version 30.0.
Results
Total 43 patients were enrolled in this study, 20 in Arm A and 23 in Arm B. 40% of Arm A and 47.83% of Arm B had left sided disease. Table 1 shows the patient and disease characteristics of the study population.
| Patient characteristics | Arm A | Arm B | p-value |
| (N= 20) | (N= 23) | ||
| Age (years) | |||
| Median | 46 | 50 | |
| Range | 39 - 59 | 26 – 58 | 0.5874 |
| Tumour grade (%) | |||
| 1 | 6 (30) | 9 (39.13) | |
| 2 | 8 (40) | 6 (26.08) | |
| 3 | 6 (30) | 8 (34.78) | 0.6166 |
| Side of primary tumour (%) | |||
| Left | 8 (40) | 11 (47.83) | |
| Right | 12 (60) | 12 (52.17) | 0.6062 |
| Histological type (%) | |||
| Infiltrating ductal | 18 (90) | 22 (95.65) | |
| Lobular | 2 (10) | 1 (4.35) | |
| Other | 0 | 0 | 0.468 |
| Pathological T staging (%) | |||
| T1a | 1 (5) | 2 (8.7) | |
| T1b | 1 (5) | 1 (4.35) | |
| T1c | 2 (10) | 1 (4.35) | |
| T2 | 1 (5) | 2 (8.7) | |
| T3 | 15 (75) | 17 (73.91) | 0.9216 |
| ER and HER2neu status (%) | |||
| ER positive, HER negative | 5 (25) | 5 (21.74) | |
| ER positive, HER positive | 7 (35) | 8 (34.78) | |
| ER negative, HER positive | 4 (20) | 7 (30.43) | |
| ER negative, HER negative | 4 (20) | 3 (13.04) | 0.8441 |
| PR status (%) | |||
| Positive | 14 (70) | 15 (65.22) | |
| Negative | 6 (30) | 8 (34.78) | 0.7385 |
| Lympho-vascular invasion (LVI) (%) | |||
| Positive | 8 (40) | 11 (47.83) | |
| Negative | 12 (60) | 12 (52.17) | 0.6062 |
| Surgical margin status (%) | |||
| Positive | 9 (45) | 9 (39.13) | |
| Negative | 11 (55) | 14 (60.87) | 0.6972 |
In a median follow-up of 42 months, only 1 (5%) patient in Arm A had chest wall recurrence, while 2 (8.7%) patients of the Arm B had the same. The comparison had no statistical significance with p-value 0.6392 (Table 2).
| Arm A | Arm B | p-value | Odds ratio | |
| (N= 20) | (N= 23) | |||
| Number of chest wall recurrence | 1 (5%) | 2 (8.7%) | 0.6392 | 0.5526 |
| No recurrence | 19 (95%) | 21 (91.3%) | 95% CI (0.0463 – 6.5953) |
Mean recurrence-free survival in Arm A was 51.150 months (standard deviation 1.624, 95% Confidence Interval = 49.526 – 52.774), and in Arm B, it was 50.633 months (standard deviation 1.828, 95% Confidence Interval = 48.805 – 52.460). In log-rank test, p-value was not statistically significant (p=0.691) (Table 3, Figure 1).
Figure 1. Kaplan-Meier Survival Analysis Curve (Arm A- Blue, Arm B- Red).
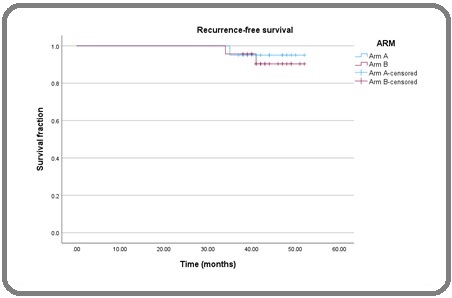
| Mean RFS (months) | Standard Deviation | 95% Confidence Interval | p-value | |
| Arm A | 51.15 | 0.828 | 49.526 – 52.774 | |
| Arm B | 50.633 | 0.932 | 48.805 – 52.460 | 0.691 |
When stratified according to the laterality of the disease, hormone receptor and HER2neu receptor status, no statistically significant difference was found (p>0.05).
Skin hyperpigmentation and ulceration were looked for as both acute and late toxicity in these patients. During treatment, 15% patients in Arm A and 17.39% of Arm B had grade 1 skin hyperpigmentation which subsided within 3 months. Grade 1 Skin ulceration was seen in 1 patient from each arm during treatment, but since the follow-up at 3 months, no patient had any ulceration. Overall, there was no statistical significance between the two arms in terms of skin toxicity (Table 4).
| Skin toxicity | Arm A (N= 20) | Arm B (N= 23) | p-value | Odds ratio |
| A. Hyperpigmentation | ||||
| During treatment (%) | ||||
| Nil | 17 (85) | 19 (82.61) | 0.8323 | 0.8382 |
| Grade I | 3 (15) | 4 (17.39) | 95% CI : 0.1636 – 4.2943 | |
| At 1 month | ||||
| Nil | 18 (90) | 21 (91.3) | 0.8833 | 1.1667 |
| Grade I | 2 (10) | 2 (8.7) | 95% CI : 0.1489 – 9.1411 | |
| At 2 months | ||||
| Nil | 20 (100) | 22 (95.65) | 0.545 | 0.3659 |
| Grade I | 0 | 1 (4.35) | 95% CI : 0.0141 – 9.4935 | |
| At 3 months | ||||
| Nil | 20 (100) | 23 (100) | 0.9462 | 1.1463 |
| Grade I | 0 | 0 | 95% CI : 0.0218 – 60.4053 | |
| B. Skin Ulceration | ||||
| During treatment (%) | ||||
| Nil | 19 (95) | 22 (95.65) | 0.9194 | 1.1579 |
| Grade I | 1 (5) | 1 (4.35) | 95% CI : 0.0677 – 19.7987 | |
| At 1 month | ||||
| Nil | 19 (95) | 22 (95.65) | 0.9194 | 1.1579 |
| Grade I | 1 (5) | 1 (4.35) | 95% CI : 0.0677 – 19.7987 | |
| At 2 months | ||||
| Nil | 20 (100) | 22 (95.65) | 0.545 | 0.3659 |
| Grade I | 0 | 1 (4.35) | 95% CI : 0.0141 – 9.4935 | |
| At 3 months | ||||
| Nil | 20 (100) | 23 (100) | 0.9462 | 1.1463 |
| Grade I | 0 | 0 | 95% CI : 0.0218 – 60.4053 |
The dose to the organs-at-risk were within the specified constraints. In Arm A, mean volume of ipsilateral lung receiving 8Gy was 12.84%, and in Arm B, mean volume of ipsilateral lung receiving 12Gy was 13.05%. For right sided breast cancer patients in Arm A, mean volume receiving 7Gy was 4.18%, and in Arm B, the mean volume receiving 10Gy was 4.21%. The same values for left breast cancer patients were 7.34% and 7.26% respectively.
No patient in either arm suffered from cough during treatment, but at 3 years of follow-up, 1 patient (5%) in Arm A and 2 patients (8.7%) in Arm B had grade 1 chronic cough. Only 1 patient in Arm A and none in Arm B suffered from grade 1 acute pneumonitis during treatment which subsided within 1 month. At 3 years, 1 patient from each arm (5% of Arm A, and 4.35% of Arm B) had developed clinically and radiologically diagnosed grade 1 pulmonary fibrosis. The difference between the two arms with respect to cough, acute pneumonitis and fibrosis occurrence was not statistically significant (Table 5).
| Pulmonary toxicity | Arm A (N= 20) | Arm B (N= 23) | p-value | Odds ratio |
| A. Cough | ||||
| During treatment (%) | ||||
| Nil | 20 (100) | 23 (100) | 0.9462 | 1.1463 |
| Grade I | 0 | 0 | 95% CI : 0.0218 – 60.4053 | |
| At 3 months | ||||
| Nil | 20 (100) | 23 (100) | 0.9462 | 1.1463 |
| Grade I | 0 | 0 | 95% CI : 0.0218 – 60.4053 | |
| At 6 months | ||||
| Nil | 19 (95) | 23 (100) | 0.4392 | 3.6154 |
| Grade I | 1 (5%) | 0 | 95% CI : 0.1393 – 93.8494 | |
| At 9 months | ||||
| Nil | 20 (100) | 23 (100) | 0.9462 | 1.1463 |
| Grade I | 0 | 0 | 95% CI : 0.0218 – 60.4053 | |
| At 12 months | ||||
| Nil | 19 (95) | 21 (91.3) | 0.6392 | 0.5526 |
| Grade I | 1 (5) | 2 (8.7) | 95% CI : 0.0463 – 6.5953 | |
| At 18 months | ||||
| Nil | 19 (95) | 21 (91.3) | 0.6392 | 0.5526 |
| Grade I | 1 (5) | 2 (8.7) | 95% CI : 0.0463 – 6.5953 | |
| At 24 months | ||||
| Nil | 18 (90) | 21 (91.3) | 0.8833 | 1.1667 |
| Grade I | 2 (10) | 2 (8.7) | 95% CI : 0.1489 – 9.1411 | |
| At 30 months | ||||
| Nil | 19 (95) | 22 (95.65) | 0.9194 | 1.1579 |
| Grade I | 1 (5) | 1 (4.35) | 95% CI : 0.0677 – 19.7987 | |
| At 36 months | ||||
| Nil | 19 (95) | 21 (91.3) | 0.6392 | 0.5526 |
| Grade I | 1 (5) | 2 (8.7) | 95% CI : 0.0463 – 6.5953 | |
| B. Pneumonitis | ||||
| During treatment (%) | ||||
| Nil | 19 (95) | 23 (100) | 0.4392 | 3.6154 |
| Grade I | 1 (5) | 0 | 95% CI : 0.1393 – 93.8494 | |
| At 3 months | ||||
| Nil | 20 (100) | 23 (100) | 0.9462 | 1.1463 |
| Grade I | 0 | 0 | 95% CI : 0.0218 – 60.4053 | |
| C. Pulmonary Fibrosis | ||||
| At 24 months | ||||
| Nil | 19 (95) | 23 (100) | 0.4392 | 3.6154 |
| Grade I | 1 (5) | 0 | 95% CI : 0.1393 – 93.8494 | |
| At 30 months | ||||
| Nil | 19 (95) | 22 (95.65) | 0.9194 | 1.1579 |
| Grade I | 1 (5) | 1 (4.35) | 95% CI : 0.0677 – 19.7987 | |
| At 36 months | ||||
| Nil | 19 (95) | 22 (95.65) | 0.9194 | 1.1579 |
| Grade I | 1 (5) | 1 (4.35) | 95% CI : 0.0677 – 19.7987 |
At the baseline, during treatment and for the first 18 months of follow-up, no patient complained of any chest pain. Mean heart doses in both arms were within the specified constraints. At 3 years, 2 patients (10%) from Arm A and 1 patient (4.35%) from Arm B had suffered from grade 1 cardiac chest pain (Angina Pectoris) but none had any myocardial infarction or cardiac arrest, and the difference between the arms had no statistical significance (Table 6).
| Cardio toxicity | Arm A (N= 20) | Arm B (N= 23) | p-value | Odds ratio |
| Cardiac chest pain (%) | ||||
| At 24 months | ||||
| Nil | 19 (95) | 22 (95.65) | 0.9194 | 1.1579 |
| Grade I | 1 (5) | 1 (4.35) | 95% CI : 0.0677 – 19.7987 | |
| At 30 months | ||||
| Nil | 19 (95) | 22 (95.65) | 0.9194 | 1.1579 |
| Grade I | 1 (5) | 1 (4.35) | 95% CI : 0.0677 – 19.7987 | |
| At 36 months | ||||
| Nil | 18 (90) | 22 (95.65) | 0.4799 | 2.4444 |
| Grade I | 2 (10) | 1 (4.35) | 95% CI : 0.2047 – 29.1900 |
All the 3 patients had left-sided disease. The mean, median and range of the ratio between Forced Expiratory Volume in 1 second and Forced Vital Capacity (FEV1/FVC ratio), and left ventricular ejection fraction (LVEF) of both arms at each assessment were compared, but no statistical significance was found.
Discussion
Studies by Yarnold et al. [17] and Owen et al. [18] showed that hypo-fractionated RT for early breast cancer may be as effective and safe as the standard 50 Gy in 25 fractions. The UK START Trial A randomized patients with early breast cancer to compare different radiotherapy regimens: 50 Gy in 25 fractions, 41.6 Gy in 13 fractions, and 39 Gy in 13 fractions, all given over 5 weeks after surgery, concluding that a lower total dose with fewer fractions could achieve comparable tumour control to standard fractionation, though the use of a conventionally fractionated boost was a significant limitation [19]. Li et al. conducted a systematic review and meta-analysis to assess the benefits of post-mastectomy radiotherapy (PMRT) in patients with T1-T2 tumours and 1-3 positive lymph nodes, finding that PMRT significantly reduced the risk of locoregional recurrence (LRR), particularly for larger tumours, though it did not significantly impact overall survival [20]. The landmark UK START Trial B randomized early breast cancer patients to receive either 50 Gy in 25 fractions or 40 Gy in 15 fractions, with a median follow-up of 6.0 years [21], finding similar effectiveness between the two regimens. Hypofractionation in breast radiotherapy became the international standard, but soon it was suggested that radiation could be given in even lesser number of fractions to improve patient compliance, reduce costs, and ease logistical challenges, as argued by Abdelmaksoud B et al [22]. Brunt et al. conducted the recent landmark FAST-Forward phase 3 clinical trial to compare a 1-week schedule with the standard 3-week schedule. In 5-year follow-up report published in 2020, they established the 26Gy/5# regimen as a safe and effective regimen [9]. Following the widespread adoption of this RT schedule in the UK, the Royal College of Radiologists Professional Support and Standards Board updated their 2016 breast radiotherapy consensus statement, making the 26 Gy/5 fractions/1 week regimen the standard for whole breast irradiation (WBI) and partial breast and chest wall radiotherapy [23].
The COVID-19 pandemic made a necessity to corroborate this data in our setting for practical purposes. Our study was conducted at Medical College Kolkata, one of the government hospitals in Eastern India with the highest footfall of patients. As most of the breast carcinoma patients attending our department are either locally advanced or metastatic, the sample size of our study was low. We did not include patients requiring nodal irradiation or boost to the tumour bed after lumpectomy. Till the date of writing up this paper, there has been no published category 1 evidence of long-term survival benefit or non-inferiority in node-positive patients. Due to lack of proper evidence, we reasonably felt no confidence to begin our institutional experience of this novel 1-week schedule on those patients, and selected a small subset of early-stage patients requiring only chest wall irradiation following modified radical mastectomy and complete axillary clearance.
43 patients were 1:1 randomized into two arms without blinding. In Arm A, among 20 patients receiving 26Gy in 5 fractions over 1 week, only 1 (5%) had chest-wall recurrence in a median follow-up period of 42 months, whereas in Arm B, among 23 patients receiving the standard 3-week regimen, the number of chest-wall recurrences was 2 (8.7%). Between the two arms, no statistically significant difference was found in recurrence-free survival or any acute or late toxicity. The dose constraints given to lungs and heart were maintained, and the late pulmonary and cardiovascular toxicities were very few in occurrence. Deep inspiratory breath-hold technique (DIBH) was not used in any of the patients due to the unavailability of breath-monitoring software.
Since we started our study, more data [24, 25] have been published supporting the FAST-Forward schedule. In 2020, Chatterjee and Chakraborty et al. started a large, multi-centre, open label, randomised controlled study across India, HYPORT-Adjuvant, to compare the 26Gy/5# schedule, with or without simultaneous integrated 6Gy/5# boost for post-breast conservation surgery patients, to the standard 40Gy/15# schedule with or without a boost of 8Gy/15# [26, 27]. In another ongoing Indian phase III non-inferiority trial, HYPART, this 26Gy/5# regimen is being compared to 34Gy/10#. They have included both lumpectomy and mastectomy patients in this study [28]. The results of these studies set in Indian setting are much anticipated.
Our study has limitations. First and foremost, the sample size was too small. In the post-hoc power analysis, the study was found underpowered. Secondly, it was a single-institutional study. Thirdly, post-breast conservation surgery (BCS) cases were not included. Fourthly, cases requiring nodal irradiation were not included in our study. Due to these factors, our findings cannot be generalized for all breast cancer patients, especially those requiring lumpectomy boost or nodal RT. We are currently conducting a large study including both PMRT and Post-BCS cases, with or without boost and nodal irradiation. We plan to follow the patients of this study for further reporting of the late toxicities. But we think our data of 3 years of follow-up in Indian patients is a valuable addition to the on-growing literature on the efficacy and the late toxicities of this 26Gy/5# ultra-hypofractionation regimen albeit the small number and subset of patients. We can safely start to prescribe this 1-week dose regimen in all post-mastectomy node-negative breast cancer patients in near future, which will be beneficial for the mostly poor patients in a governmental tertiary cancer centre setup in terms of significant decrease in the cost of travelling, the period of staying at the hospital, loss of daily wages, and mental stress.
In conclusion, there was no statistically significant difference observed between the 1-week schedule (26Gy in 5 fractions) and the standard 3-week regimen of adjuvant radiotherapy in post-mastectomy, node-negative, early-stage breast cancer patients in terms of local control, acute and late skin, pulmonary and cardiac toxicities.
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for- profit sectors.
The authors declare no conflict of interest.
References
- Breast cancer Loibl S, Poortmans P, Morrow M, Denkert C, Curigliano G. Lancet (London, England).2021;397(10286). CrossRef
- Five-year results of a prospective case series of accelerated hypofractionated whole breast radiation with concomitant boost to the surgical bed after conserving surgery for early breast cancer Cante D, Franco P, Sciacero P, Girelli G, Marra AM , Pasquino M, Russo G, et al . Medical Oncology (Northwood, London, England).2013;30(2). CrossRef
- Effect of radiotherapy after breast-conserving surgery on 10-year recurrence and 15-year breast cancer death: meta-analysis of individual patient data for 10,801 women in 17 randomised trials Darby S., McGale P., Correa C., Taylor C., Arriagada R., Clarke M., Cutter D., et al . Lancet (London, England).2011;378(9804). CrossRef
- Effect of radiotherapy after mastectomy and axillary surgery on 10-year recurrence and 20-year breast cancer mortality: meta-analysis of individual patient data for 8135 women in 22 randomised trials McGale P., Taylor C., Correa C., Cutter D., Duane F., Ewertz M., Gray R., et al . Lancet (London, England).2014;383(9935). CrossRef
- Radiation therapy for operable breast cancer: sixty years of progress as seen through the articles published in the journal Cancer Freedman G. M.. Cancer.2008;113(7 Suppl). CrossRef
- The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials Haviland JS , Owen JR , Dewar JA , Agrawal RK , Barrett J, Barrett-Lee PJ , Dobbs HJ , et al . The Lancet. Oncology.2013;14(11). CrossRef
- Long-term results of hypofractionated radiation therapy for breast cancer Whelan TJ , Pignol JP , Levine MN , Julian JA , MacKenzie R, Parpia S, Shelley W, et al . The New England Journal of Medicine.2010;362(6). CrossRef
- Ten-Year Results of FAST: A Randomized Controlled Trial of 5-Fraction Whole-Breast Radiotherapy for Early Breast Cancer Brunt AM , Haviland JS , Sydenham M, Agrawal RK , Algurafi H, Alhasso A, Barrett-Lee P, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2020;38(28). CrossRef
- Hypofractionated breast radiotherapy for 1 week versus 3 weeks (FAST-Forward): 5-year efficacy and late normal tissue effects results from a multicentre, non-inferiority, randomised, phase 3 trial Murray Brunt A, Haviland JS , Wheatley DA , Sydenham MA , Alhasso A, Bloomfield DJ , Chan C, et al . Lancet (London, England).2020;395(10237). CrossRef
- OC-0101 First results of FAST-Forward phase 3 RCT nodal substudy: 3-year normal tissue effects | Request PDF Wheatley D , Haviland J , Patel J , Sydenham M , Alhasso A , Chan C , et al . Radiother Oncol.2022;170:S75–6. CrossRef
- European Society for Radiotherapy and Oncology Advisory Committee in Radiation Oncology Practice consensus recommendations on patient selection and dose and fractionation for external beam radiotherapy in early breast cancer Meattini I, Becherini C, Boersma L, Kaidar-Person O, Marta GN , Montero A, Offersen BV , et al . The Lancet. Oncology.2022;23(1). CrossRef
- Hypofractionation: The standard for external beam breast irradiation Brunt AM , Haviland JS . Breast (Edinburgh, Scotland).2023;69. CrossRef
- Breast Cancer Atlas https://www.rtog.org/CoreLab/ContouringAtlases/BreastCancerAtlas.aspx .
- Common Terminology Criteria for Adverse Events (CTCAE) Version 5.0. https://ctep.cancer.gov/protocoldevelopment/electronic_applications/docs/ctcae_v5_quick_ref erence_5x7.pdf. .
- Nonparametric statistical tests for the continuous data: the basic concept and the practical use Nahm FS . Korean Journal of Anesthesiology.2016;69(1). CrossRef
- Statistical notes for clinical researchers: Chi-squared test and Fisher's exact test Kim HY . Restorative Dentistry & Endodontics.2017;42(2). CrossRef
- Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial Yarnold J, Ashton A, Bliss J, Homewood J, Harper C, Hanson J, Haviland J, Bentzen S, Owen R. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2005;75(1). CrossRef
- Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial Owen JR , Ashton A, Bliss JM , Homewood J, Harper C, Hanson J, Haviland J, Bentzen SM , Yarnold JR . The Lancet. Oncology.2006;7(6). CrossRef
- The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial Bentzen S. M., Agrawal R. K., Aird E. G. A., Barrett J. M., Barrett-Lee P. J., Bliss J. M., Brown J., et al . The Lancet. Oncology.2008;9(4). CrossRef
- Post-mastectomy radiotherapy for breast cancer patients with t1-t2 and 1-3 positive lymph nodes: a meta-analysis Li Y, Moran MS , Huo Q, Yang Q, Haffty BG . PloS One.2013;8(12). CrossRef
- The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial Bentzen S. M., Agrawal R. K., Aird E. G. A., Barrett J. M., Barrett-Lee P. J., Bentzen S. M., Bliss J. M., et al . Lancet (London, England).2008;371(9618). CrossRef
- Postmastectomy Hypofractionated Irradiation in Egyptian patients with Breast Cancer: Zagazig University Experience Abdelmaksoud BA , et al . Int J Cancer Oncol.2018;5(1):8-12.
- on behalf of the Breast Radiotherapy Consensus Working Group. Moving forward fast with FAST-Forward Lewis P, Brunt AM , Coles C, Griffin S, Locke I, Roques T. Clin. Oncol.2021;33:427-429.
- Ultra-Hypofractionation for Whole-Breast Irradiation in Early Breast Cancer: Interim Analysis of a Prospective Study Sigaudi V, Zannetti M, Ferrara E, Manfredda I, Mones E, Loi G, Krengli M, Franco P. Biomedicines.2022;10(10). CrossRef
- Ultra-hypofractionated one-week locoregional radiotherapy for patients with early breast cancer: Acute toxicity results Ratosa I, Montero A, Ciervide R, Alvarez B, García-Aranda M, Valero J, Chen-Zhao X, et al . Clinical and Translational Radiation Oncology.2024;46. CrossRef
- HYPOfractionated Radiation Therapy comparing a standard radiotherapy schedule (over three weeks) with a novel one week schedule in Adjuvant breast cancer: An open-label randomised controlled study (HYPORT- Adjuvant): study protocol for a multicenter, randomized phase III trial Chatterjee S, Chakraborty , Santam & Group , HYPORT . 2020. CrossRef
- HYPORT adjuvant acute toxicity and patient dosimetry quality assurance results - Interim analysis Chakraborty S, Chatterjee S. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2022;174. CrossRef
- HYPofractionated Adjuvant RadioTherapy in 1 versus 2 weeks in high-risk patients with breast cancer (HYPART): a non-inferiority, open-label, phase III randomised trial Yadav BS , Dahiya D, Kannan P., Goyal S, Laroiya I, Irrinki S, Singh NR , Sharma R. Trials.2024;25(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details