Association of Estrogen Receptor TA Repeats with Endometrial and Ovarian Cancers in Basrah Province
Download
Abstract
Background: Ovarian and endometrial malignancies are complex diseases, since a defect in hormone balance can be the most important cause of tumor formation.
Aim of study: Examine and identify the association between ER TA repeats with endometrial and ovarian cancers in Basrah women.
Patients: Groups include 50 healthy controls and 50 cancer patients, divided into 20 endometrial cancer patients and 30 ovarian cancer patients.
Results and Discussion: The result shows TA 11–12 repeats are the most common in endometrial cancer in 68% of cancer patients, followed by TA≥10 in 8% and 4% for both 13–14 repeats and ≤15, whereas TA repeats 11–12 and ≤15 repeats are the most common in ovarian cancer in 22% for each one, followed by ≥10 TA in 10% and 13–14 in 6%. The control groups have four groups of repeat numbers: 11–12 TA repeat in 68%, 13–14 in 20%, ≤15in 10%, and ≥10 in 2%. Simultaneously, a substantial association exists between the two types of cancers and the TA repeat length variation of the estrogen receptor type alpha.
Conclusion: The length of the TA repeat in estrogen receptor alpha has a significant risk for ovarian and endometrial cancer.
Introduction
Endometrial cancer (EC) is a prevalent type of cancer in Europe, and the infection is managed by professionals from different health specializations. Nowadays, significant progress has been achieved in the treatment of endometrial cancer patients in the fields of molecular biology and minimally invasive surgery. In 2020, 417.367 cases were recorded and 100.000 deaths, so the ratio increased with aging and rising obesity rates among people in high-income countries [1]. Surgery is the first choice in the early stages, followed by a care follow-up for many years.
The defect in hormone balance is considered the major cause of EC infection. The estrogen signaling caused by estrogen receptor α (ER α) acts as a carcinogenic indication in some cases, leading to special treatment to control this signal during infection. In spite of the fact that there is a correlation between estrogen signaling and EC cases, the function of ER through molecular aspects is still poorly identified; however, the research progresses, increasing the understanding between them [2].
Ovarian cancer (OC) is the most prevalent malignancies after cervix and uterine, since it is asymptomatic until it spread due to more than two-thirds of patients already have advanced illness at the time of diagnosis. Ovarian cancer is one of the major complexes in the field of surgery, which requires serious and complex treatment and depletes the patient’s mental and physical energy, leading to the highest death rates among all female genital malignancies [3, 4]. The five years cause-specific survival ranges from (20%) in stage four and (40%) in stage three to (70%) in stage two and (90%) in stage one [5].
Fifteen to twenty percent of patients contain germline mutations, even though over eighty percent of cases are sporadic and lack a documented genetic tendency. A significant DNA damage response (DDR) system, homologous recombination repair (HRR) pathways, are affected in half of the patients by mutations in these genes [6].
Hormonal imbalance is one of the many potential contributing factors to the development of ovarian cancer.
A class of hormones known as estrogens is involved in both physiological and pathological processes. They may control the propagation, invasion, and conversion of epithelial to mesenchymal in OC patients. Additionally involved in controlling the biology of the tumor microenvironment is estrogen signaling [7].
Estrogen (E), is a steroid hormone contain estradiol and estrone, which mostly secreted by the ovaries. It plays a vital function in many organs especially in ovaries, testes, uterus, and the endometrium, by stimulating the changes in the endometrium through the periodic changes [8]. Fallowed with mammary glands and skeletal muscles also in cardiovascular and immune systems [9-11].
The estrogen receptor (ER) is a group of proteins in the cell that gives them the ability to absorb estrogen which is a group of female hormones from the blood stream of a fertile woman. The ER is divided into two groups: the intracellular nuclear receptor family comprises the nucleus estrogen receptors, ERα and Erβ, as well as the membrane estrogen receptors, ER-X, GPER, and Gq-mER, which are G protein-coupled receptors [12]. The ESR1 gene is responsible for coding ERα at locus 6q25.1 [13, 14]. There is a large isoform (66 kDa), in addition to multiple shorter ones of ERα (36 kDa, 46 kDa), leading to the presence of alternative starting codons or being identified as another graft product (Figure 1).
Figure 1. Structure of Estrogen Receptor α and Their Isoforms. The domain arrangement of the full-length 595 amino acid ERα (67kDa) and truncated shorter isoforms (62kDa, 53kDa, 46kDa, 45kDa, and 36kDa) resulting from alternative splicing and/or alternate translation initiation sites is illustrated. Protein domains are labeled A through F, with the number indicating the amino acid sequence number based on the full-length protein (595 aa). ERα domains include N-terminal (NTD, A/B domains, AF-1), DNA binding domain (DBD, C domain), hinge (D) domain, and C-terminal region containing the ligand binding domain (LBD, E/F domain, AF-2).
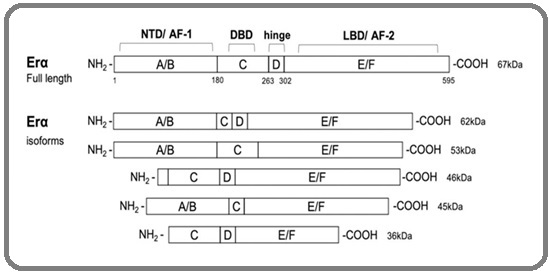
Some of these shorter isoforms lack the NTD , leading to a lack of the AF-1 domain that enables transcription. Rather, they can join together to form heterodimers with full-length ERα, preventing it from controlling transcription. After binding to estretrol, estriol, and estradiol, the shorter isoform, ERα-36, has been demonstrated to start signaling events through the membrane. It does this by lacking the transcriptional activation domains of both AF-1 and AF-2 [15].
The study aimed to examine and identify the association between ER TA repeats with endometrial and ovarian cancers in Basrah women.
Materials and Methods
Samples Collection
Fifty of blood samples were collected from endometrial and ovarian cancer patients from Basrah Oncology and Hematology Center, their age range between (37 to 80) years old. On the other hand, fifty blood samples of female with no cancer were collected as a control group their ages between (27 to 75) years old. Two ml of peripheral blood was drawn by sterilized syringe from the two groups then they have been kept in sterilized EDTA tubes for DNA extraction.
Extraction of DNA from peripheral blood
Genomic DNA from peripheral blood was extracted according to the instructions provided with genomic DNA mini kit (Geneaid, Taiwan). The isolated DNA was examined by agarose gel electrophoresis (0.8%).
Amplification of TA repeats in Estrogen receptor gene
The fragment was amplified using forward primer 5`-GCAGAATCAAATATCCAGATG-3` and reverse 5`-GACGCATGATATACT’TCACC-3` by using 12.5 μl of master mix (Promega, USA), 1 μl from forward and reverse primers (Pioneer, Korea), 2 μl of template DNA and 8.5 μl of nuclease free water (Bioneer, Korea) than the mixture mixed and subjected to thermocycler (Bioneer, Korea) using the following program: denaturation at 94ºC, annealing at 58ºC and extension at 72ºC for 35 sec. to each step [16]. The amplified fragments detected by 2% agarose gel electrophoresis when the fragment ranges from 140 to 160bp. Fifteen μl of PCR product were subjected to Macrogen company in South Korea for sequencing. The sequences were processed and analyzed using Basic Local Alignment Search Tool BLAST’ to search for homologous sequences in the National Center for Biotechnology Information database [17].
Statistical Analysis
Descriptive statistics have been used to describe patient’s characteristics by using percentage. ORs and the 95 % CLs were calculated online, the value considered significant when OR≥ 1.5. Also Q square was used to evaluate significant differences for some parameters.
Results
Amplification of target fragments
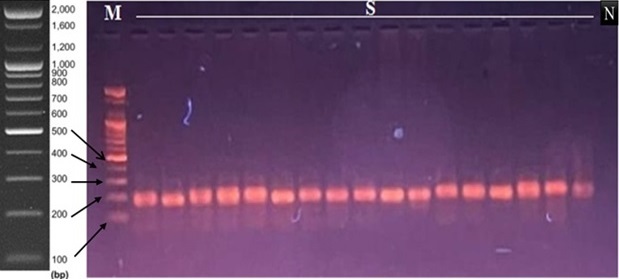
The accuracy and integrity of PCR for ERα were determined using agarose gel electrophoresis, where the band was seen and documented at 140–160 bp on the agarose gel (Figure 2).
Figure 2. Agarose gel Electrophoresis of the ERα Gene. The amplification bands are observed at 140-160 bp. M; 100 bp DNA marker, S; samples, N; negative control.
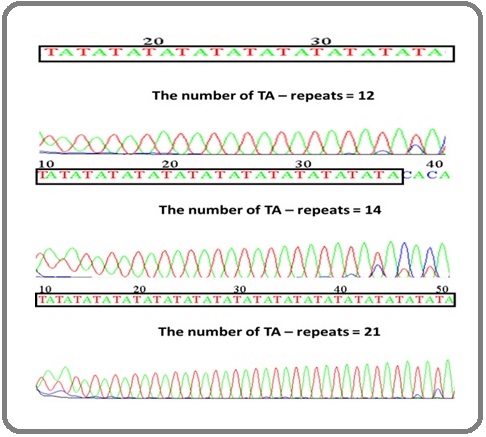
Sequencing of the ERα gene in control and patient The frequency of TA repeats for ERα amplicon was determined by the sequence of the fragment, as shown in (Figure 3), which shows the variance length of TA repeats.
Figure 3. The Sequence of ERα Gene in Some Studied Subjects. The TA-repeats length polymorphic region was marked..
Frequency of TA-repeats length polymorphism of ERα gene
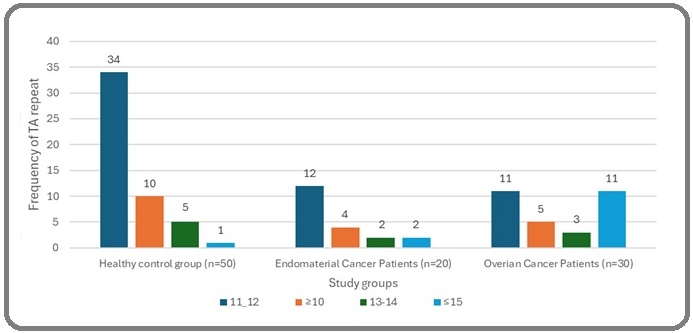
The frequency of TA repeats in ERα gene was assessed depending on the sequence analysis that divided the individuals into four groups, including: a- TA repeats ≤10; b- TA repeats of 11–12 repeats; c- TA repeats of 13–14 repeats; and d- TA repeats of ≥15. In healthy controls, the length of TA repeats ranged from 10 to 20 repeats.
Most of the control individuals had 11–12 TA repeats (68%), followed by people with 13–14 TA repeats (20%) and people have ≤15 (20%) .While only one sample (2%) belonged to group (a), which had 10 TA repeats. On the other hand, most patients had 11–12 repeats (46%), followed by patients with TA repeats of ≤15 (26%), TA lengths of ≥ 10 and 13–14 repeats, which were (18%-10%), respectively.
The comparison between ovarian and endometrial cancer according to TA repeats showed that the most prevalent groups in ovarian cancer patients are 11–12 and ≤15 repeats in 11 out of 30 patients for each group, while the most prevalent groups in endometrial cancer are TA repeats lengths of 11–12 with a frequency of 12 out of 20 (Figure 4).
Figure 4. Frequency of Different TA-repeats Length Polymorphisms of ERα Gene in the Studied Population.
Association of ERα length polymorphism with endometrial cancer
A significant correlation was found between the two variables when the length of TA-repeats was compared in the control and endometrial populations. In other words, statistical analysis revealed a relation between the length of TA-repeats and a higher risk of EC (p=0.048). Furthermore, it came to light that patients with TA-repeat count of fewer than 10 had an estimated 11.33-fold higher risk of EC than those in the healthy control group (repeat counts of 11–12). The other TA-repeat length did not, however, appear to be correlated with a higher risk of EC. A statistical analysis of endometrial cancer risk and the ERα TA-repeats length variation is presented in (Table 1).
| TA-repeats | Control group N=(50) | EC Patients N=(20) | OR | 95% CI | p-value |
| 11-12 | 34 (68%) | 12 (24%) | 1 | ------- | - |
| ≥10 | 1 (2%) | 4 (8%) | 11.33* | 1.15 - 111.692 | 0.013 |
| 13-14 | 10 (20%) | 2 (4%) | 0.57 | 0.108 - 2.964 | 0.497 |
| ≤15 | 5 (10%) | 2 (4%) | 1.13 | 0.194 - 6.633 | 1 |
OR, Odds ratio; 95 % CI, 95 % confidence interval; * statistically significant
Association of ERα length polymorphism and grade of EC
The statical analysis showed that no significant association (p=0.18) between the grade of endometrial cancer and the variation of TA repeats length in spite of most of the patients (80%) had grade I-II as shown in (Table 2).
| TA-repeats | Grade I-II | Grade III-IV | OR | 95% CI |
| 11-12 | 8 (40%) | 4 (20%) | 1 | ------- |
| ≥10 | 4 (20%) | 0 | ||
| 13-14 | 4 (20%) | 0 |
χ2,3.33; p,0.18
Association of ERα length polymorphism and ovarian cancer
The chi-square test revealed a substantial difference (p=0.001) in TA repeat length distribution in OC, indicating a possible correlation between TA repeats and OC. According to the risk estimate analysis, patients with TA-repeat lengths of ≥10 and ≤ 15 had a 15.45 and 6.8 fold higher risk of OC, respectively, compared to the reference group (11–12 repeats), as shown in (Table 3).
| TA-repeats | Control group N=(50) | OC Patients N=(30) | OR | 95% CI | p-value |
| 11-12 | 34 (68%) | 11 (22%) | 1 | ------- | - |
| 10≥ | 1 (2%) | 5 (15%) | 15.45* | 1.625 - 146.938 | 0.003 |
| 13-14 | 10 (20%) | 3 (6%) | 0.92 | 0.216 - 3.986 | 1 |
| 15≤ | 5 (10%) | 11 (22%) | 6.8* | 1.935 -23.898 | 0.001 |
χ2, 16.6; p,0.001*; OR = Odds ratio; 95 % CI = 95 % confidence interval; * statistically significant
Association of ERα length polymorphism and grades of ovarian cancer
There is no significant association between the TA- repeat length and grades and OC (p=0.21), as found in (Table 4).
| TA-repeats | Grade I-II | Grade III-IV | OR | 95% CI | p-value |
| 11-12 | 6 | 5 | 1 | ------- | |
| 10≥ | 5 | 0 | --- | ------- | |
| 13-14 | 2 | 1 | 0.6 | 0.041 - 8.732 | 0.706 |
| 15≤ | 5 | 6 | 1.44 | 0.269 -7.714 | 0.669 |
χ2,4.49; p,0.21
Discussion
Endometrial and ovarian cancers are two common tumors among women worldwide, leading to around 340000 and 140000 deaths annually [3,18]. As with many other cancer types, these ones may have a number of environmental and genetic variables linked to their onset and progression. Consequently, the researchers are looking at the reasons that intrinsic and extrinsic risk factors play in the development of ovarian and endometrial malignancies. To that end, we sought to define in this work the impact of genetic polymorphisms on the occurrence of ovarian and endometrial malignancies in a group of women from Basrah, Iraq.
The estrogen role is recorded in many types of cancer, including breast, prostate, and endometrial. One of the reasons for that is that the genetic alteration affects the expression level of estrogen, increasing the chance of developing cancer. For example, estrogen has an effective role in the proliferation of the cells of endometrial cancer [19].
A rare and limited study on the role between the polymorphism in the promoter of Erα and cancer, one of these studies in the Swedish women population shows that there is a strong linkage between the length of TA- repeats in ERα and endometrial cancer, and that the short repeat is linked significantly with disease [20]. All of the previous studies agreed with the present research because there is a strong correlation between the length of repeats and EC. Additionally, patients with TA repeats of ≥10 had an estimated 11.33-fold higher risk of ovarian cancer.
Intron I SNPs in the ERα gene promoter are in linkage disequilibrium with the upstream TA repeat polymorphism [21]. As a result, the genetic polymorphism in the proximal region of the promoter affects the regulation of the transcription of these genes, and the TA repeat number may be affecting the transcription [22].
The promoter of ERα gene have multiple functions including regulation of gene transcription, the TA length polymorphism located between promoter A and B effect on promoter function and the transcription [23]. As previously mentioned, the change in the expression of ERα may lead to the formation and progression of more than one type of cancer.
Cancer of the ovary is a significant worldwide health issue because of the delayed detection and absence of an effective treatment plan [18]. Since there is no proven and trustworthy method for screening for ovarian cancer, pre-screening high-risk patients based on their genetic and epidemiological traits is taken into consideration [18]. While certain screening measures are available, such as transvaginal ultrasonography and the measurement of the biomarker CA125, they are not thought to be useful prognostic tools for the condition and have not yet shown significant improvements in patient mortality [24]. An essential role for estrogen in ovarian cancer leads to a significant link between ovarian cancer and ERα TA length polymorphism.
Additionally, the number of TA repeats doesn’t have a link with the grades of ovarian and endometrial cancers; in other words, the previous polymorphism doesn’t have a role in the disease severity.
In conclusion, the length of the TA repeat in estrogen receptor alpha has a significant risk for ovarian and endometrial cancer, while the TA length doesn’t have a role in the grades of these cancers.
Ethical approval
All participants in the study were informed and provided explicit verbal consent. The work was approved by the Committee on Publication Ethics at Basrah Oncology and Hematology Center.
Conflict of interest
No conflict of interest.
References
- Management of Patients Diagnosed with Endometrial Cancer: Comparison of Guidelines Restaino S, Paglietti C, Arcieri M, Biasioli A, Della Martina M, Mariuzzi L, Andreetta C, et al . Cancers.2023;15(4). CrossRef
- Estrogen Signaling in Endometrial Cancer: a Key Oncogenic Pathway with Several Open Questions Rodriguez AC , Blanchard Z, Maurer KA , Gertz J. Hormones & Cancer.2019;10(2-3). CrossRef
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- Cancer statistics, 2023 Siegel RL , Miller KD , Wagle NS , Jemal A. CA: a cancer journal for clinicians.2023;73(1). CrossRef
- Ovarian cancer statistics, 2018 Torre LA , Trabert B, DeSantis CE , Miller KD , Samimi G, Runowicz CD , Gaudet MM , Jemal A, Siegel RL . CA: a cancer journal for clinicians.2018;68(4). CrossRef
- Treatment of Ovarian Cancer Beyond PARP Inhibition: Current and Future Options Garg V, Oza AM . Drugs.2023;83(15). CrossRef
- Estrogens, Estrogen Receptors and Tumor Microenvironment in Ovarian Cancer Kozieł MJ , Piastowska-Ciesielska AW . International Journal of Molecular Sciences.2023;24(19). CrossRef
- Estrogen Biosynthesis and Signal Transduction in Ovarian Disease Xu X, Huang Z, Yu K, Li J, Fu X, Deng S. Frontiers in Endocrinology.2022;13. CrossRef
- Functions of estrogen and estrogen receptor signaling on skeletal muscle Ikeda K, Horie-Inoue K, Inoue S. The Journal of Steroid Biochemistry and Molecular Biology.2019;191. CrossRef
- Estrogen Receptor Signaling in the Immune System Chakraborty B, Byemerwa J, Krebs T, Lim F, Chang C, McDonnell DP . Endocrine Reviews.2023;44(1). CrossRef
- Research Progress of Estrogen Receptor in Ovarian Cancer Zhang M, Xu H, Zhang Y, Li Z, Meng W, Xia J, Lei W, Meng K, Guo Y. Clinical and Experimental Obstetrics & Gynecology.2023;50(9). CrossRef
- Molecular mechanism of estrogen-estrogen receptor signaling Yaşar P, Ayaz G, User SD , Güpür G, Muyan M. Reproductive Medicine and Biology.2017;16(1). CrossRef
- Human estrogen receptor beta-gene structure, chromosomal localization, and expression pattern Enmark E., Pelto-Huikko M., Grandien K., Lagercrantz S., Lagercrantz J., Fried G., Nordenskjöld M., Gustafsson J. A.. The Journal of Clinical Endocrinology and Metabolism.1997;82(12). CrossRef
- Estrogen Receptors Mediated Negative Effects of Estrogens and Xenoestrogens in Teleost Fishes-Review Wojnarowski K, Cholewińska P, Palić D, Bednarska M, Jarosz M, Wiśniewska I. International Journal of Molecular Sciences.2022;23(5). CrossRef
- The therapeutic target of estrogen receptor-alpha36 in estrogen-dependent tumors Gu Y, Chen T, López El, Wu W, Wang X, Cao J, Teng L. Journal of Translational Medicine.2014;12. CrossRef
- Estrogen receptor alpha dinucleotide repeat and cytochrome P450c17alpha gene polymorphisms are associated with susceptibility to endometriosis Hsieh Y, Chang C, Tsai F, Lin C, Tsai C. Fertility and Sterility.2005;83(3). CrossRef
- Association of TTTA polymorphism in CYP19 gene with endometrial and ovarian cancers risk in Basrah Ayyob AN , Al-Badran AI , Abood RA . Gene Rep.2019;16:100453. CrossRef
- Ovarian cancer Matulonis UA , Sood AK , Fallowfield L, Howitt BE , Sehouli J, Karlan BY . Nature Reviews. Disease Primers.2016;2. CrossRef
- Estrogen Enhances Endometrial Cancer Cells Proliferation by Upregulation of Prohibitin Yang B, Chen R, Liang X, Shi J, Wu X, Zhang Z, Chen X. Journal of Cancer.2019;10(7). CrossRef
- Estrogen receptor alpha gene polymorphisms and endometrial cancer risk Weiderpass E., Persson I., Melhus H., Wedrén S., Kindmark A., Baron J. A.. Carcinogenesis.2000;21(4). CrossRef
- A TA repeat polymorphism in the estrogen receptor gene is associated with osteoporotic fractures but polymorphisms in the first exon and intron are not Langdahl B. L., Løkke E., Carstens M., Stenkjaer L. L., Eriksen E. F.. Journal of Bone and Mineral Research: The Official Journal of the American Society for Bone and Mineral Research.2000;15(11). CrossRef
- VNTR sequence on human chromosome 11p15 that affects transcriptional activity Iwashita S, Koyama K, Nakamura Y. Journal of human genetics.2001;46(12). CrossRef
- Estrogen receptor gene polymorphisms and the genetics of osteoporosis: a HuGE review Gennari L., Merlotti D., De Paola V., Calabrò A., Becherini L., Martini G., Nuti R.. American Journal of Epidemiology.2005;161(4). CrossRef
- Ovarian cancer screening and mortality in the UK Collaborative Trial of Ovarian Cancer Screening (UKCTOCS): a randomised controlled trial Jacobs IJ , Menon U, Ryan A, Gentry-Maharaj A, Burnell M, Kalsi JK , Amso NN , et al . Lancet (London, England).2016;387(10022). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details