Celiac Disease and Cancer: Epidemiological Evidence, Diagnostic Challenges, and Future Research Directions
Download
Abstract
Celiac disease (CD) is an autoimmune condition affecting multiple systems of the body. It is triggered in people with specific genetic profiles upon eating gluten. Current estimates suggest that about 0.5 to 1 percent of the global population is affected. Failure to follow a gluten-free diet (GFD) in these patients can cause side effects such as resistant CD. Various studies have reported that failure to follow a GFD increases the risk of diseases such as cancer. This review analyzed prevailing theories on epidemiological findings, diagnostic complexities, and avenues for further investigation. In the first part of the study, the relationship between CD and types of cancer was discussed, which showed that, based on previous studies, gastrointestinal cancers and intestinal lymphoma are most closely related to CD. Also, the frequency of ovarian cancer, breast cancer, and colon cancer in CD is significantly lower. In the next part of the study, the relationship between CD in children and types of cancer in this age group was analyzed based on studies. The results showed that intestinal lymphoma and thyroid cancer are the most common types of cancer in children. Also, in the next part of this study, the GFD’s effect on cancer types was examined. The studies reviewed in this section showed that in some cases, a GFD can reduce the risk of cancer. In another part of this study, cancer diagnosis methods in CD were minimally examined. The results showed that one of the critical challenges in celiac patients is the timely and accurate diagnosis of cancer in them, which can reduce mortality and even improve the treatment of these patients. However, these views need further investigation, and this review study cannot be sufficient to prove such a theory. To continue this study, descriptive or cohort research is recommended to investigate the association between CD and GFD and the prevalence of various cancers in these patients in a larger population.
Introduction
Celiac disease (CD) is a disorder of the immune system impacting roughly 1 in 100 people, with the worldwide rate of diagnosed cases on the rise. Research has placed the overall frequency of the disease somewhere between 0.7 percent and 1.4 percent, solidly marking the breadth of the condition across diverse populations and suggesting a wider, undetermined, undiagnosed reservoir [1-4]. Some studies have also shown that the incidence of celiac disease in different populations should be much lower than the actual estimate due to late diagnosis or lack of diagnosis of this disease [5]. CD triggers an immune response that leads to swelling of the small intestine lining and causes the tiny projections called villi to shrink when the diet includes gluten-containing foods [6]. For people with CD, eating gluten can trigger symptoms like long-lasting diarrhea, sharp stomach pain, swelling, and even noticeable weight loss [6]. CD can also be divided into several types based on the severity and symptoms it causes [4, 7]. Some people have classic CD, which has symptoms such as bloating, diarrhea, and weight loss, and this type of disease is more common in children [8-10]. In another type, the disease is seen as symptomatic, and this type of CD usually has no digestive symptoms. Still, symptoms include fatigue, iron deficiency, vitamin deficiency, and abdominal pain. In other people, the disease may also occur as silent celiac, in which the person does not experience any symptoms and is diagnosed incidentally with serological tests. In another case, the disease shows celiac antibody blood tests, but the intestinal biopsy may be negative. In rare cases, another type of celiac disease may occur in a person. In this type of disease, the person follows a gluten-free diet but does not improve. This is called treatment-resistant celiac disease, and in this case, the doctor prescribes steroid drugs for treatment [11-13]. CD is usually more common in people with a weaker immune system. Most people with CD carry either the HLA-DQ2 or HLA-DQ8 gene variant [14]. Most of the inflammation is produced in these people due to the gut response to the gluten-derived protein gliadin found in wheat, barley, and rye. This hypersensitivity increases the lamina propria thickness, minor intestinal epithelium inflammation, and damage to the intestinal villi, which are gradually flattened and finally atrophy. In these immunological processes, the innate and the adaptive immune systems play a significant role in activating gliadin-reactive T cells, macrophages, lymphocytes, and dendritic cells. CD has gastrointestinal and extragastrointestinal symptoms. The only treatment currently available for celiac patients is strict GFD [15, 16]. Various studies have shown that a gluten-free diet can improve clinical symptoms and disease course. Removing gluten from the diet is very difficult because wheat is used in most foods, which reduces the quality of life of these patients [17]. Also, some patients who follow a gluten-free diet may experience more severe symptoms due to the presence of some malignancies, such as cancer, which may even cause the death of the patients. Patients with celiac disease have high levels of inflammatory cytokines, which can cause chronic inflammation in these patients [18]. Prolonged inflammation in people with CD can increase the likelihood of several gastrointestinal cancers. Studies have shown strong associations between celiac disease and all cancers of the digestive system, such as cancer of the oesophagus, colon, intestinal lymphoma, small intestine, and stomach [19, 20]. The risk of developing these cancers in undiagnosed celiacs is significantly increased, but this can be reversed by early diagnosis of the disease and treatment with a gluten-free diet [21]. Patients with celiac disease may also have a suppressed immune system, and these iron and vitamin B12 deficiencies could increase the cancer risk. This compromised immune response impairs their capacity to fight cancer cells [22]. Therefore, people with CD are at high risk of developing cancer. According to various studies, a precise relationship between CD and cancer has not yet been determined. In this study, several theories will be examined. In the first part of the study, what is the relationship between CD and types of cancer, and which cancers are most closely related to celiac disease? In the next part of the study, the relationship between celiac disease in children and types of cancer at this age will be briefly explained. Will those who have had CD since childhood have a higher percentage of cancer? In the next part of this review, what effect does a gluten-free diet have on the risk of developing types of cancer in these patients? In some cases, it has been determined that a gluten-free diet can reduce the risk of cancer. Also, in the other part of the study, cancer diagnosis methods in celiac patients will be examined minimally, because one of the important challenges in celiac patients is the timely and accurate diagnosis of cancer in them, which can reduce mortality and even improve the treatment of these patients. This study also aims to investigate the relationship between CD and cancer [22] (Figure 1).
Figure 1. Schematic Representation of the Relationship between Celiac Disease (CD) and Cancer Risk .
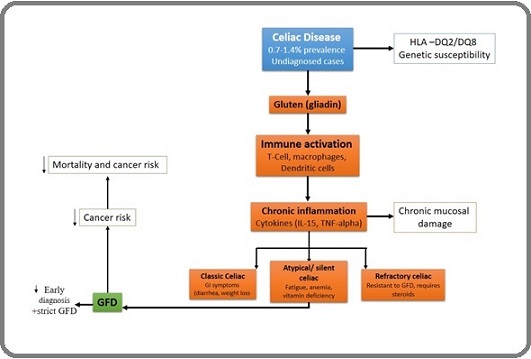
Many studies have shown that celiac disease can cause many malignancies in patients; some malignancies, such as various cancers, can cause severe symptoms in patients with celiac disease. In this part of our study, cancers that can be most related to celiac disease were collected and categorized from various studies. According to multiple studies, it has been shown that small intestine lymphoma, Esophageal Cancer (EC), Colorectal Cancer (CRC), and small intestine adenocarcinoma (SBA) have been reported most frequently in patients with celiac disease. Still, cancers such as primary liver cell, breast, Oropharyngeal (OPC), Endometrial, and Ovarian (OC) have had little relationship with CD. Also, in this part of the study, for a simple review by researchers interested in this topic, cancers related to celiac disease are schematically shown in Figure 2.
Figure 2. A Brief Overview of Cancers Associated with Celiac Disease. All the cancers related to celiac disease are described in more detail in this section .
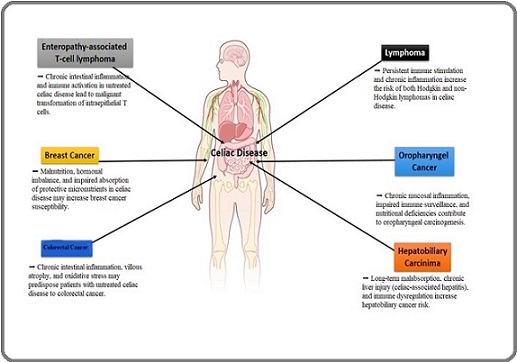
In this section, on the other hand, cancers reported in patients with celiac disease will be explained in detail.
Esophageal cancer (EC)
Esophageal cancer (EC) is the seventh most common cancer among men. It is highly prevalent in men due to alcohol consumption, smoking, and poor nutrition. In addition, the risk of developing this type of disease may increase due to factors such as Crohn’s disease and Gastroesophageal reflux [23, 24]. According to studies, the prevalence of esophageal cancer in Iran, India, and China is much higher than in other countries. Some studies have observed that patients with CD are at risk of developing EC. It has been found that delayed gastric emptying in patients with CD is one of the main factors in these patients developing EC [25]. Also, abnormal levels of hormones related to the digestive system in undiagnosed celiac patients can be a factor in the development of EC. Continuous reflux of acid from the stomach into the esophagus causes damage to the esophagus and, as a result, causes EC in these patients in the long term. Using a GFD is one of the main strategies to reduce this type of damage in patients with CD [5].
Colorectal Cancer (CRC)
It is a cancer that occurs in the colon. It is the third most common cancer in men and the second most common cancer in women. The risk of developing this type of cancer may increase with age, poor nutrition, and lifestyle. In addition to these factors, family history also plays a role in increasing the risk of developing this type of cancer [26]. This cancer begins with the deletion of the adenomatous polyposis coli (APC) gene located on chromosome 5q21, the activation of the KRAS oncogene gene, and a mutation in the TP53 gene. A study conducted by Lasa et al. in 2018 showed that CD can increase the risk of CRC [27]. Also, a survey conducted by Onwuzo et al. in 2023 examined the increased risk of CRC in patients with CD. This study analyzed about 47,400,960 patients with CD [26]. They showed that the risk of CRC was significant in people with CD. They also concluded that celiac patients were more likely to develop CRC even after taking into account risk factors. This study also showed that the effect of CD is not limited to the small intestine but can also involve other parts of the digestive tract, such as the CRC [26]. Continuous exposure of CD patients to gluten-containing foods can increase the risk of CRC. Contrary to previous reports, other studies have not reported any significant association between CD and an increased risk of CRC. People with CD usually have a low BMI compared to healthy people. This factor can be one of the protective factors in measuring the risk of CD [17].
In this section, some studies have reported an increased risk, but others have reported a protective effect or no association in their results. These discrepancies in results could be due to differences in study design, screening for colorectal cancer at different time periods, duration and intensity of adherence to gluten-free treatment, and genetic and epidemiological differences in the populations studied. Also, the available evidence is not conclusive, and a clinical trial study with careful control of adherence to a gluten-free diet is needed to achieve better results.
Small Intestine Adenocarcinoma (SBA)
This type of cancer is a rare neoplasm that occurs in less than 5% of gastrointestinal cancers. It affects all three parts of the digestive tract, the duodenum, jejunum, and ileum. The cancer starts in the lining of the SBA and then spreads to deeper parts [28]. The genes that are implicated in this type of cancer include: TP53, KRAS, APC, SMAD4, BRAF, SOX9, ATM, ARID2, ACVR2A,
ACVR1B, BRCA2, and SMARCA4 [29]. In patients with celiac disease, the leading cause of this type of cancer can be inflammation of the small intestine due to gluten consumption. This factor causes inflammation of the small intestinal villi. In CD, gliadin peptides cause the release of zonulin, which disrupts the intestinal epithelial barrier [30]. Therefore, persistent intestinal inflammation and cell morphological changes can increase the risk of developing SBA cancer. In a study conducted by Oliveira et al. in 2025 [31], a case of SBA in a patient with CD was reported. They showed that this disease may have symptoms very similar to CD, and the researchers of this study also emphasized that the patient should follow a GFD when symptoms are present. Given the association of this type of cancer with CD, it is essential to evaluate for SBA when the patient with CD does not respond to a GFD [31].
Lymphoma
It is the most common type of cancer in children aged 15 to 19. This type of lymphoma accounts for about 5% of gastrointestinal lymphomas [32]. The pattern of occurrence of this type of cancer varies considerably based on age, sex, geographic location, and even lifestyle. Patients with CD are at high risk of developing lymphoma. Untreated CD patients can be at risk of developing enteropathy-associated T-cell lymphoma (EATL) [33, 34]. This type of disease is divided into two types. Type 1. This disease is prevalent in patients with CD [34]. Type 1 EATL may be more common in people who do not consume gluten. Studies have shown that using a gluten-free diet to treat CD can prevent the development of EATL in them [34]. In a survey conducted by Martin- Masot et al. in 2023, it was shown that CD could be a risk factor for developing lymphoma. In this study, eleven non-HLA SNPs were selected and investigated. The results of the study reported a significant association between CD and lymphoma. These findings provide new insights into the relationship between immune intolerance and the development of EATL in patients with CD [35].
The evidence and results of this part of the study are strong and proven, so almost all clinical and epidemiological studies have confirmed the association between CD and EATL. The minor discrepancies seen in the studies are due to late diagnosis or clinical error. Avoiding gluten consumption and early diagnosis of patients can prevent the high incidence of this type of lymphoma.
Primary liver cell cancer/Hepatocellular Carcinoma (HCC)
This disease is the most common type of HCC. It is the seventh most common type of cancer in women and the fifth most common type of cancer in men. It is the second leading cause of death. Risk factors for this disease include smoking, alcohol, and a history of underlying diseases such as CD and fatty liver [36]. Celiac hepatitis is one of the most common forms of liver abnormalities in patients with CD. Untreated CD can cause an imbalance in liver enzymes and ultimately HCC. The primary mechanism by which patients with CD develop HCC is not yet known [11]. However, there are several hypotheses in this regard, one of which is a change in the intestinal microbiota. CD usually causes a decrease in the intestinal microbiota, which can increase the risk of liver cancer/HCC in these patients [8]. Also, gluten consumption in patients with CD can cause multiple inflammatory responses and consequently cause liver damage, which can also cause liver cirrhosis and ultimately liver cancer/HCC. In general, it can be said that untreated CD can increase the risk of liver cancer/ HCC [37].
Most studies have reported an increased risk for this type of cancer. The differences in the studies’ results may be due to the small sample size and the rarity of this type of cancer, which limits statistical validity. Adding CD to the routine screening program for this type of cancer can help early detection of the disease.
Studies conducted on this type of cancer need further confirmation and investigation. Also, the reasons for the different results could be differences in microbiota dysbiosis and the presence of other underlying factors, such as hepatitis or alcohol consumption. Finally, and in general, the role of microflora in the liver’s precancerous mechanism should be further investigated.
Breast Cancer (BC)
According to the World Health Organization, breast cancer (BC) is the most common type of cancer among women. Risk factors for breast cancer include age, sex, diet, hormonal status, lifestyle, and family history [38, 39]. A study conducted by Askling et al. in 2002 showed that patients with CD have a very low risk of BC [40]. This study attributed the low risk of BC in patients with CD to lactose intolerance in celiac patients who consume fewer dairy products [41]. Also, women with CD have a low nutritional status due to intestinal villi malabsorption, which causes them to lose weight, which could be one of the factors that reduces the risk of BC in these patients. Another study also showed that the reduced risk of BC in CD patients is due to their low BMI [5].
Oropharyngeal Cancer (OPC)
This type of cancer is the eighth most common type of cancer in men. This cancer usually develops in the scrotum. This type of disease risk factors include human papillomavirus, alcohol consumption, and smoking [42]. A study has shown that the risk of developing oral and OPC is increased in patients with CD [43]. In a survey by Askling et al. in 2002, 11,019 patients with CD were examined [40]. The results of the study showed that patients with CD are several times more at risk of developing cancer of the pharynx, mouth, and esophagus [44]. Studies have also shown that people who have a GFD and those who have a regular diet have an increased risk of oral, pharynx, and esophageal cancer [44]. Also, a study conducted by Holmes et al. in 1989 showed that a GFD can be protective against these types of malignancies [45].
Endometrial Cancer
This cancer is the fourth most common type of cancer in women. There are two types of uterine cancer: one is estrogen-dependent, and the other is estrogen-independent. The most common type of uterine cancer is estrogen-dependent uterine cancer [38]. Studies have shown that this type of cancer is inversely related to CD. This type of cancer is usually very rarely reported in patients with CD [17]. Studies have shown that CD can cause premature menopause and, as a result, reduce estrogen levels in women, which can be a risk factor for uterine cancer. Therefore, according to studies, CD can have a negative relationship with uterine cancer in women. Patients with CD who have experienced premature menopause can be treated with a GFD [46].
Ovarian Cancer (OC)
This cancer is the seventh most common type of cancer among women. Studies have reported that the risk of OC in patients with CD is very low [38]. This is because people with CD are less exposed to estrogen throughout their lives. Estrogen has a direct link to OC [5, 47].
Most studies on the association between CD and breast, ovarian, and thyroid cancers have found no significant association, but some reports have shown a protective effect. The reasons for these differences in study results could include the effects of malnutrition and reduced BMI. In general, large multicenter studies are needed to examine this association more closely.
The main mechanisms of the association between celiac disease (CD) and cancer
Based on the results of existing studies, the association between CD and various types of cancer cannot be fully explained by presenting statistics and study results alone. Instead, the biological and immunological pathways that cause this association should be examined. These pathways, which are well summarized in Table 1, can provide an analytical framework for interpreting the various results.
1. Chronic inflammation and the cytokine network
Continuous gluten consumption in people with CD causes their innate and adaptive immune systems to be constantly activated (increased interleukin 15, IFN-γ, TNF-α). This factor creates a tumor microenvironment associated with oxidative stress, DNA damage, and gene mutations. Cancers such as enteropathy-associated T-cell lymphoma (EATL) and small intestinal adenocarcinoma are closely related to this pathway.
2. Genomic instability and impaired DNA repair
Defects in mismatch repair pathways and oxidative stress cause microsatellite instability and mutations in the TP53 and JAK/STAT genes, which can contribute to developing colorectal cancers and some lymphomas.
3. Microbiota dysbiosis and carcinogenic metabolites
Changes in the normal flora of patients with CD can increase metabolite products and systemic inflammation, which increases the risk of liver cancers and other gastrointestinal cancers in these individuals.
4. Nutritional deficiency and immunosuppression
Chronic malabsorption of iron, folic acid, and vitamin B12 reduces the ability of cells to suppress the antitumor immune system and repair DNA. This factor can increase some cancers, and has a protective effect for some cancers, such as breast and ovarian cancer.
By analyzing each cancer type in terms of these pathways, causal inconsistencies in studies can be explained. For example, discrepancies in reports of colorectal cancer risk could be due to differences in gluten-free diets or the severity of chronic inflammation in the populations studied.
Relationship between celiac disease (CD) and cancers
The association between celiac disease and various cancers has been established for several years. Fairley and Mackie were the first to report a patient with malignant lymphoma of the small intestine and steatorrhea in 1937 [48]. By 1961, intestinal malabsorption was considered a symptom of malignant small intestine lymphoma. In 1962, Gough et al. were able to report 5 cases of small intestinal lymphoma in patients with celiac disease. This study showed that celiac disease is a malignant disease [49].
Also, a study in the United Kingdom showed that malignant lymphoma of the small intestine is the cause of death in patients with celiac disease. In general, these studies showed that cancers associated with celiac disease are high and include cancers such as adenocarcinoma of the mouth, pharynx, and small intestine, as well as lymphoma of the small intestine [50-52]. O’Farrelly et al. in 1986 introduced the term “enteropathy-associated T-cell lymphoma” (EATL), which may be specifically associated with CD [53]. CD treatment with a gluten-free diet, which may also prevent malignancy, was also suggested by a retrospective study 1980 [53]. The association of cancer with CD has received much attention. However, it is essential to note that most of these studies have been very limited due to the small number of cancers analyzed (Figure 3).
Figure 3. Studying the Immunological and Molecular Mechanisms of Digestive Cancers and Their Relationship with Celiac Disease (CD).
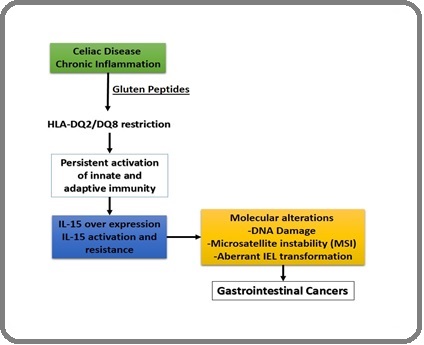
Therefore, such analyses may have overestimated the association of cancer with CD. In a study by Askling et al. in Sweden in 2002, 11,019 patients with CD were analyzed. Of these, 8012 were under 19 years of age, a very low incidence of malignancy in this group. This study was excluded after the first year of CD diagnosis, when most cancer diagnoses are made [40]. Two studies in the United Kingdom also showed a very low risk and no cancer risk in CD [54, 55]. Elfstrom et al. reported no increased risk of cancer in patients with CD [1]. In a study conducted by Goerres et al. in 2006, they showed that UDP-glucuronosyltransferases, which are involved in the detoxification of toxins and carcinogens, are reduced in CD, which could increase the risk of cancer in these patients [14, 56]. Also, Kamycheva et al. in 2017 showed that leukocyte telomere length in celiac patients indicates genomic instability, which could also increase the risk of cancer in these patients [57]. Another study conducted by Demiroren et al. in 2022 showed that resistant CD with abnormal intraepithelial lymphocytes can be considered prelymphoma [14]. Also, a study conducted by Gromny et al. in 2023 showed that the association between pancreatic cancer and CD is still unclear, and further studies are needed to provide more detailed information about this association [6]. Another study by KALRA et al. in 2022 showed that an increased risk of gastrointestinal cancer could be associated with CD, and they also showed that the increased risk of colon cancer in patients with CD is minimal. It was also shown that the use of a gluten-free diet can have a positive effect on reducing the risk of cancer in these people [5]. Also, a study by Somtochukwu Onwuzo et al. in 2023 showed that most patients with CD develop colon cancer even when considering their risk factors [26]. Another cohort study by Malamut et al. in 2025 showed that screening for small bowel cancer in patients with CD is recommended by colonoscopy. This study strongly emphasizes the importance of colonoscopy in celiac patients. The researchers of this study also showed that following a gluten-free diet can positively reduce the risk of small bowel cancer (Table 1 and Table 2) [58].
| Mechanism | Molecular/Immunological Events | Role in Carcinogenesis |
| Chronic Inflammation | Persistent gluten-driven activation of innate (IL-15, IFN-γ) and adaptive (CD4+ T cells, cytotoxic CD8+ IELs) immunity | Creates a pro-tumorigenic microenvironment via oxidative stress, cytokine storm, and DNA damage |
| Interleukin-15 (IL-15) Overexpression | IL-15 drives activation and survival of intraepithelial lymphocytes (IELs), resistance to apoptosis | Promotes clonal expansion of aberrant IELs → progression to EATL |
| Loss of Immune Tolerance | Breakdown of tolerance to gluten peptides and cross-reactive autoantigens | Sustained T-cell activation → epithelial injury → compensatory proliferation (risk of mutation) |
| DNA Damage and Microsatellite Instability (MSI) | Oxidative stress + defective mismatch repair pathways | Accumulation of mutations in oncogenes (e.g., MYC, JAK/STAT) and tumor suppressors (e.g., TP53) |
| Aberrant IEL Transformation | Expansion of CD3+CD8+ IELs with activating mutations (e.g., JAK1, STAT3, SETD2) | Leads to refractory celiac disease type II (RCD-II) and progression to EATL |
| Cytokine Network | High levels of TNF-α, IFN-γ, and IL-21 in the intestinal mucosa | Supports epithelial apoptosis, compensatory hyperplasia, and neoplastic transformation |
| Epigenetic Changes | DNA methylation alterations and histone modification in chronic inflammation | Epigenetic silencing of tumor suppressor genes → oncogenic signaling |
| Microbiota Dysbiosis | Altered gut flora in CD with chronic inflammation | May contribute to pro-carcinogenic metabolites and epithelial stress |
| Nutritional Deficiencies (e.g., Folate, Iron) | Chronic malabsorption → impaired DNA repair capacity | Increased risk of mutagenesis |
| Year | Study (First author; Journal) | Country/Design | Cancer type(s) | Estimate (95% CI) | Key notes | Source |
| 2002 | Askling J; Gastroenterology | Sweden; nationwide cohort (hospitalized CD/DH) | All cancers (adults); Colon carcinoma | 1.3 (overall, adults); 1.9 (1.2-2.8) for colon | Increased risk was also reported for the small intestine, oropharyngeal, esophageal, hepatobiliary, and pancreatic; decreased breast cancer risk was mentioned in later literature. | Askling 2002 (Gastroenterology); colon SIR via later meta-analysis reporting [40] |
| 2003 | Green PHR; Am J Med | USA; clinic-based cohort | Non-Hodgkin lymphoma (overall) | increased vs. general population (exact summary varies by subgroup) | Early evidence of elevated lymphoma risk in CD: a small sample clinic cohort | Green 2003 (Am J Med) [64] |
| 2004 | West J; BMJ | UK; population-based GP database cohort | Any malignancy; Gastrointestinal; Lymphoproliferative; Breast; Lung | Any: 1.29 (1.06-1.55); GI: 1.85 (1.22-2.81); Lymphoproliferative: 4.80 (2.71-8.50); Breast: 0.35 (0.17-0.72); Lung: 0.34 (0.13-0.95) | Excess risk is concentrated in the first year after diagnosis; after excluding the first year, any malignancy is HR 1.10 (0.87- 1.39). | West 2004 (BMJ) [55] |
| 2011 | ElfstrÃm P; J Natl Cancer Inst | Sweden; nationwide histology-based cohort | Lymphoproliferative malignancy (overall); by histology severity | Overall LPM HR ~2.8; Persistent villous atrophy SIR 3.78 vs. healed mucosa SIR 1.50 | Risk of LPM is highest with persistent villous atrophy at follow-up biopsy. | ElfstrÃm 2011 (JNCI) & companion analysis [65] |
| 2012 | ElfstrÃm P; Clin Gastroenterol Hepatol | Sweden; nationwide histology-based cohort | Any gastrointestinal (esophagus→anus, incl. hepatobiliary & pancreas in sensitivity analyses) | After first year: 1.07 (0.93-1.23); First year markedly elevated (short-term ascertainment) | Absolute excess risk for GI cancer ≠2 per 100,000 person-years. | ElfstrÃm 2012 (CGH) [1] |
| 2020 | Emilsson L; Gastroenterology | Sweden; nationwide cohort | Small bowel adenocarcinoma; Small bowel adenoma; Carcinoid | SBA: 3.05 (1.86-4.99); Adenoma: 5.73 (4.11-7.99); Carcinoid: 1.14 (0.72-1.81) | Most substantial relative risks observed for small bowel adenoma/adenocarcinoma. | Emilsson 2020 (Gastroenterology) [1] |
| 2022 | Lebwohl B; Clin Gastroenterol Hepatol | Sweden; nationwide histology-based cohort (47,241 CD) | Any cancer; site-specific signals (hematologic, lymphoproliferative, hepatobiliary, pancreatic) | Any cancer: 1.11 (1.07-1.15); First year: 2.47 (2.22-2.74); After first year: 1.01 (0.97-1.05); Breast: 0.83 (0.74-0.92); Lung: 0.88 (0.75-1.03) | Overall, excess is confined mainly to the first year after diagnosis, with persistent elevation for selected sites. | Lebwohl 2022 (CGH) [59] |
Diagnose types of cancers in patients with celiac disease (CD)
Diagnosing types of cancer in patients with CD requires precise and targeted strategies, as CD can cause chronic intestinal inflammation, which can increase the risk of developing types of cancer such as EATL and other cancers, especially digestive cancers [42]. Diagnostic methods in these conditions include biopsy, serological tests, endoscopy, and genetic and molecular studies. In the first stage, the doctor may suspect cancer based on symptoms such as weight loss, bloating, abdominal pain, and lack of response to a GFD and request tests such as CBC, inflammatory marker tests such as ESR and CRP, and for CD, tests such as checking the level of tTG-IgA and DGP-IgG antibodies. CT scans and MRIs are also performed to examine abnormal masses in the abdomen and pelvis [59-61]. Also, in cases of suspected lymphoma, a biopsy of the small intestine (duodenum or jejunum) is necessary [60]. Table 3 summarizes cancer diagnosis cases in patients with CD, which may be very useful and practical for some researchers in this field to diagnose suspected cases in patients with CD better.
| Method | Application in Celiac Disease Patients | Strengths | Limitations | Clinical Notes |
| Endoscopy (Upper GI endoscopy, Colonoscopy, Capsule endoscopy, Enteroscopy) | Detects mucosal lesions (ulcers, strictures, tumors); Capsule valuable endoscopy for small bowel screening | Direct visualization; Can obtain targeted biopsies; Capsule enables complete small bowel assessment. | Capsule: risk of retention in strictures; Misses submucosal lesions; Colonoscopy limited to colon/terminal ileum. | First-line for suspected malignancy in CD (esp. small bowel adenocarcinoma, lymphoma, EATL) |
| Histological Biopsy | Gold standard for confirming both celiac disease (villous atrophy) and cancer (adenocarcinoma, lymphoma) | Definitive tissue diagnosis; Enables immunohistochemistry and molecular testing | Sampling error possible; Requires expertise; May miss patchy lesions | Essential for differentiating EATL from reactive lymphocytosis; repeat biopsy if suspicion persists |
| Imaging (CT, MRI, PET-CT, Ultrasound) | Staging and detection of extraluminal or advanced disease (lymph nodes, metastasis) | CT/MRI show mass lesions, staging; PET-CT detects metabolically active lymphoma; US for l iver/biliary lesions | Less sensitive for small mucosal lesions; Radiation exposure (CT, PET); Cost (PET, MRI) | Used after endoscopic suspicion/biopsy confirmation for staging and monitoring |
Diagnosing malignancies in patients with celiac disease (CD), particularly in the context of chronic inflammation and ongoing intestinal injury, presents unique challenges. A major difficulty lies in differentiating enteropathy-associated T-cell lymphoma (EATL) from refractory CD, as both may manifest with similar features such as persistent diarrhea, significant weight loss, and severe malnutrition. Under conditions of chronic inflammation, endoscopic and histopathological findings can be non-specific and difficult to interpret. Imaging pitfalls are a significant concern: inflamed or fibrotic bowel sections can resemble mass lesions on CT or MRI, or hide small cancerous spots. While PET/ CT provides better sensitivity, active inflammation can cause false positives, so it is important to interpret results carefully alongside clinical information and additional tests. Clinically, if a CD patient shows no response or experiences secondary deterioration following remission, this should prompt suspicion of malignancy. A systematic diagnostic process should include:
Confirming strict adherence to a gluten-free diet through serology and nutritional assessment.
Repeat endoscopy with multiple-site small bowel biopsies.
Using both cross-sectional imaging (CT/MRI) and functional imaging (PET/CT) together to identify potential lesions.
Immunophenotyping of tissue samples to differentiate EATL from other lymphomas or persistent inflammatory changes.
This layered approach enhances the chances of early detection and can considerably improve clinical outcomes.
The link between cancer and children with celiac disease (CD)
Evidence on cancer risk in children with celiac disease is limited and sometimes contradictory. Several small studies have indicated a higher rate of intestinal lymphoma and thyroid cancer among pediatric CD patients. Still, these results are not conclusive due to small sample sizes and short follow-up periods [59]. Therefore, caution is essential when interpreting these data, as cancer in children with CD appears uncommon overall. However, persistent, refractory, or undiagnosed CD might increase the risk of neoplastic changes, especially in lymphoid tissues and the thyroid. This topic would benefit from being part of a broader discussion on lymphoma in CD, considering age-related differences and disease manifestations. Future studies should focus on enrolling pediatric patients in long-term, multicenter research to determine better the actual cancer risk in this group [26].
Because no researcher has yet examined this connection in childhood, this section could be critical and interesting for many researchers. I want to start the discussion on this topic by asking the question: Do those who develop CD in childhood have an increased risk of developing certain types of cancer? In an international study by the Spanish Society for Pediatric Gastroenterology, Hepatology and Nutrition (ESPGHAN), 22 children with CD and cancer were evaluated [17]. The studies showed intestinal lymphoma and thyroid carcinoma were more common than expected. Still, other researchers have not yet confirmed these findings, so this research needs to be confirmed by other researchers [62].
Association between gluten-free diet (GFD) and various cancers
Several studies have examined the effects of a gluten-free diet on improving mucosal damage caused by CD [5]. Studies show that following a GFD in CD completely improves mucosal inflammation in these patients [16]. However, few studies have examined the effects of a GFD on reducing cancer in these patients. In a survey conducted by Kalra et al. in 2022, they examined the impact of a GFD on various types of malignancies, such as cancer. In this study, which was conducted as a review, they concluded that a GFD in patients with CD can positively reduce the risk of cancer in these patients [5]. Also, in a book published in 2023 by Botosso et al., they discussed the role of a GFD in causing malignancies such as cancer in patients with CD [63]. Their study stated that no precise scientific relationship has yet been seen regarding the relationship between a GFD in patients with CD and reducing cancer in them [63]. However, another critical study conducted by Holmes et al. in 1989 showed that the incidence of oral, pharyngeal, and esophageal cancers was much lower in celiac patients who had been on a GFD for a long time [45]. It has also been shown that patients with refractory CD may develop histopathological lesions when exposed to gluten, which may also cause severe immunological responses in these patients. On the other hand, non-adherence to GFD in CD can cause chronic inflammation of the small intestine and increase inflammatory signals in these patients, ultimately leading to the onset of various lymphomas and malignant cancers [16].
In conclusion, a review of various studies shows that the association between CD and some cancers, such as intestinal lymphoma and small intestinal adenocarcinoma, is relatively confirmed. At the same time, there are conflicting results regarding colorectal and esophageal cancers. These discrepancies can be attributed to differences in sample size, study design, method, duration of adherence to gluten-free diet treatment, and changes in diagnostic procedures. Given the significant progress, the current knowledge is still insufficient to assess the risk in various cancers accurately; future studies should focus on studies with larger sample sizes, analysis of clinical factors, lifestyle, and investigation of molecular and immunological mechanisms. Such methods could lead to practical preventive and therapeutic approaches for celiac patients.
Acknowledgments
This work was supported by the Celiac Disease and Gluten-Related Disorders Research Center, Research Institute for Gastroenterology and Liver Diseases, Shahid Beheshti University of Medical Sciences, Tehran, Iran. It has not received specific funding from the public, commercial, or non-profit sectors.
Conflict of Interest
The authors declare no conflict of interest.
References
- Low risk of gastrointestinal cancer among patients with celiac disease, inflammation, or latent celiac disease Elfström P, Granath F, Ye W, Ludvigsson JF . Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association.2012;10(1). CrossRef
- Epidemiology of Celiac Disease Myleus A, Catassi C. Gastrointestinal Endoscopy Clinics.2025;0(0). CrossRef
- Global prevalence of ultra-short coeliac disease (USCD): the first systematic review and meta-analysis Karimzadhagh S, Ahmadi B, Olfatifar M, Houri H, Asri N, Rostami-Nejad M, Sanders DS . Gut.2025;74(7). CrossRef
- The Global Epidemiology of Celiac Disease: How Often is Celiac Disease Diagnosed, and Has This Changed Over Time? King JA . 2019.
- Current updates on the association between celiac disease and cancer, and the effects of the gluten‑free diet for modifying the risk (Review) Kalra N, Mukerjee A, Sinha S, Muralidhar V, Serin Y, Tiwari A, Verma AK . International Journal of Functional Nutrition.2022;3(1). CrossRef
- Pancreatic Cancer in Celiac Disease Patients-A Systematic Review and Meta-Analysis Gromny I, Neubauer K. International Journal of Environmental Research and Public Health.2023;20(2). CrossRef
- Coeliac disease Catassi C, Verdu EF , Bai JC , Lionetti E. Lancet (London, England).2022;399(10344). CrossRef
- Liver Involvement in Celiac Disease and Immune-Mediated Diseases of the Small Bowel Nandi N, Verdu EF , Schuppan D, Gomez-Aldana AJ , Pinto-Sanchez MI , Conforti FS , Maggioni M, et al . Liver International: Official Journal of the International Association for the Study of the Liver.2025;45(8). CrossRef
- Celiac disease: a comprehensive current review Caio G, Volta U, Sapone A, Leffler DA , De Giorgio R, Catassi C, Fasano A. BMC medicine.2019;17(1). CrossRef
- Geographic trends and risk of gastrointestinal cancer among patients with celiac disease in Europe and Asian-Pacific region Rostami Nejad M, Aldulaimi D, Ishaq S, Ehsani-Ardakani MJ , Zali MR , Malekzadeh R, Rostami K. Gastroenterology and Hepatology from Bed to Bench.2013;6(4).
- Long-term risk of chronic liver disease in patients with celiac disease: a nationwide population-based, sibling-controlled cohort study Yao J, Sun J, Ebrahimi F, Bergman D, Green PHR , Hagström H, Lebwohl B, Leffler DA , Ludvigsson JF . The Lancet Regional Health. Europe.2025;50. CrossRef
- Celiac disease. Textbook of pediatric gastroenterology, hepatology and nutrition: A comprehensive guide to practice: Springer Guandalini S, Discepolo V. 2021;:525-48.
- Evaluating CD4 and Foxp3 mRNA Expression in Tissue Specimens of Celiac Disease and Colorectal Cancer Patients Asri N, Nazemalhosseini Mojarad E, Taleghani MY , Houri H, Saeedi Niasar M, Rezaei-Tavirani M, Jahani-Sherafat S, et al . Asian Pacific journal of cancer prevention: APJCP.2024;25(2). CrossRef
- Possible relationship between refractory celiac disease and malignancies Demiroren K. World Journal of Clinical Oncology.2022;13(3). CrossRef
- Association of celiac disease and intestinal lymphomas and other cancers Catassi C, Bearzi I, Holmes GKT . Gastroenterology.2005;128(4 Suppl 1). CrossRef
- The link between gluten intake and the risk of cancers Bakhtiari S, Asri N, Maleki S, Rahimi S, Jabbari A, Ahmadzadeh A, Jahani-Sherafat S, et al . Gastroenterology and Hepatology from Bed to Bench.2024;17(2). CrossRef
- Association Between Celiac Disease and Cancer Marafini I, Monteleone G, Stolfi C. International Journal of Molecular Sciences.2020;21(11). CrossRef
- Association between intestinal neoplasms and celiac disease: A review Wang M, Yu M, Kong W, Cui M, Gao F. World Journal of Gastrointestinal Oncology.2021;13(9). CrossRef
- Cancer risk in celiac disease Loftus CG , Loftus EV . Gastroenterology.2002;123(5). CrossRef
- Expression of tolerogenic dendritic cells in the small intestinal tissue of patients with celiac disease Kheiri F, Rostami-Nejad M, Amani D, Sadeghi A, Moradi A, Aghamohammadi E, Sahebkar A, Zali MR . Heliyon.2022;8(12). CrossRef
- Association between coeliac disease and gastrointestinal cancers: a narrative review Montorsi RM , Esposito A, Pastena MD . Digestive Medicine Research.2023;6(0). CrossRef
- Delayed diagnosis of coeliac disease increases cancer risk Silano M, Volta U, Mecchia AM , Dessì M, Di Benedetto R, De Vincenzi M. BMC gastroenterology.2007;7. CrossRef
- Oesophageal cancer and gastro-oesophageal reflux: what is the relationship? Lagergren J. Gut.2004;53(8). CrossRef
- The association between celiac disease and digestive system cancers: A Mendelian randomization study Yuan C. 2024.
- Celiac Disease and Esophageal Cancer Khoshnia M, M, Islami F, Aghcheli K, Malekzadeh R, Pourshams A. Govaresh.2012;17(2):73-7.
- Increased Risk of Colorectal Cancer in Patients With Celiac Disease: A Population-Based Study Onwuzo S, Boustany A, Saleh M, Gupta R, Onwuzo C, Mascarenhas Monteiro J, Lawrence F, et al . Cureus.2023;15(3). CrossRef
- Colorectal Adenoma Risk Is Increased among Recently Diagnosed Adult Celiac Disease Patients Lasa J, Rausch A, Bracho LF , Altamirano J, Speisky D, Dávila MTG , Iotti A, Zubiaurre I. Gastroenterology Research and Practice.2018;2018. CrossRef
- Small bowel adenocarcinoma as a complication of celiac disease: clinical and diagnostic features Caio G, Volta U, Ursini F, Manfredini R, De Giorgio R. BMC gastroenterology.2019;19(1). CrossRef
- Small bowel adenocarcinomas in celiac disease follow the CIM-MSI pathway Bergmann F, Singh S, Michel S, Kahlert C, Schirmacher P, Helmke B, Von Knebel Doeberitz M, et al . Oncology Reports.2010;24(6). CrossRef
- Small bowel adenocarcinoma complicating coeliac disease: a report of three cases and the literature review Benhammane H, El M'rabet FZ , Idrissi Serhouchni K, El Yousfi M, Charif I, Toughray I, Mellas N, et al . Case Reports in Oncological Medicine.2012;2012. CrossRef
- A Case Report of Celiac Disease and Small Bowel Adenocarcinoma: Two Diseases With Similar Symptoms Oliveira A, Gonçalves G, Paracana B, Baptista L, Rodrigues T. Cureus.2025;17(4). CrossRef
- Enteropathy associated T cell lymphoma in celiac disease: a large retrospective study Malamut G, Chandesris O, Verkarre V, Meresse B, Callens C, Macintyre E, Bouhnik Y, et al . Digestive and Liver Disease: Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver.2013;45(5). CrossRef
- Risks for lymphoma and gastrointestinal carcinoma in patients with newly diagnosed adult-onset celiac disease: Consequences for follow-up: Celiac disease, lymphoma and GI carcinoma Gils T, Nijeboer P, Overbeek LI , Hauptmann M, Castelijn DA , Bouma G, Mulder CJ , Leeuwen FE , Jong D. United European Gastroenterology Journal.2018;6(10). CrossRef
- Patients with celiac disease and B-cell lymphoma have a better prognosis than those with T-cell lymphoma Halfdanarson TR , Rubio-Tapia A, Ristow KM , Habermann TM , Murray JA , Inwards DJ . Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association.2010;8(12). CrossRef
- Celiac Disease Is a Risk Factor for Mature T and NK Cell Lymphoma: A Mendelian Randomization Study Martín-Masot R, Herrador-López M, Navas-López VM , Carmona FD , Nestares T, Bossini-Castillo L. International Journal of Molecular Sciences.2023;24(8). CrossRef
- Celiac disease and risk of liver disease: a general population-based study Ludvigsson JF , Elfström P, Broomé U, Ekbom A, Montgomery SM . Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association.2007;5(1). CrossRef
- Prevalence of Celiac Disease in Patients With Liver Diseases: A Systematic Review and Meta-Analyses Yoosuf S, Singh P, Khaitan A, Strand TA , Ahuja V, Makharia GK . The American Journal of Gastroenterology.2023;118(5). CrossRef
- Reduced risk of breast, endometrial and ovarian cancer in women with celiac disease Ludvigsson JF , West J, Ekbom A, Stephansson O. International Journal of Cancer.2012;131(3). CrossRef
- The 2019 World Health Organization classification of tumours of the breast Tan PH , Ellis I, Allison K, Brogi E, Fox SB , Lakhani S, Lazar AJ , et al . Histopathology.2020;77(2). CrossRef
- Cancer incidence in a population-based cohort of individuals hospitalized with celiac disease or dermatitis herpetiformis Askling J, Linet M, Gridley G, Halstensen TS , Ekström K, Ekbom A. Gastroenterology.2002;123(5). CrossRef
- The Risk of Malignancies in Celiac Disease-A Literature Review Pelizzaro F, Marsilio I, Fassan M, Piazza F, Barberio B, D'Odorico A, Savarino EV , Farinati F, Zingone F. Cancers.2021;13(21). CrossRef
- Malignancies in Patients with Celiac Disease: Diagnostic Challenges and Molecular Advances Ivanova M, Bottiglieri L, Sajjadi E, Venetis K, Fusco N. Genes.2023;14(2). CrossRef
- Oral Manifestations Leading to a Diagnosis of Celiac Disease: A Case Report Vargo DRJ . Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology.2024;138(2). CrossRef
- Coeliac disease and malignancy Holmes G. K. T.. Digestive and Liver Disease: Official Journal of the Italian Society of Gastroenterology and the Italian Association for the Study of the Liver.2002;34(3). CrossRef
- Malignancy in coeliac disease--effect of a gluten free diet Holmes G. K., Prior P., Lane M. R., Pope D., Allan R. N.. Gut.1989;30(3). CrossRef
- Risk of endometriosis in 11,000 women with celiac disease Stephansson O, Falconer H, Ludvigsson JF . Human Reproduction (Oxford, England).2011;26(10). CrossRef
- The Relation between Celiac Disease and Ovarian Reserve: A Case-Control Study Mahmoudi N, Besharat S, Pichak M, Livani S. International Journal of Fertility & Sterility.2025;19(3). CrossRef
- Clinical and Biochemical Syndrome in Lymphadenoma Fairley N. H., Mackie F. P.. British Medical Journal.1937;1(3972). CrossRef
- Intestinal reticulosis as a complication of idiopathic steatorrhoea Gough K. R., Read A. E., Naish J. M.. Gut.1962;3(3). CrossRef
- Coeliac disease, gluten-free diet, and malignancy Holmes G. K., Stokes P. L., Sorahan T. M., Prior P., Waterhouse J. A., Cooke W. T.. Gut.1976;17(8). CrossRef
- Coeliac disease and malignancy Swinson C. M., Slavin G., Coles E. C., Booth C. C.. Lancet (London, England).1983;1(8316). CrossRef
- Mortality in celiac disease Logan R. F., Rifkind E. A., Turner I. D., Ferguson A.. Gastroenterology.1989;97(2). CrossRef
- Humoral response to wheat protein in patients with coeliac disease and enteropathy associated T cell lymphoma O'Farrelly C., Feighery C., O'Briain D. S., Stevens F., Connolly C. E., McCarthy C., Weir D. G.. British Medical Journal (Clinical Research Ed.).1986;293(6552). CrossRef
- Risk of malignancy in diagnosed coeliac disease: a 24-year prospective, population-based, cohort study Card T. R., West J., Holmes G. K. T.. Alimentary Pharmacology & Therapeutics.2004;20(7). CrossRef
- Malignancy and mortality in people with coeliac disease: population based cohort study West J, Logan RFA , Smith CJ , Hubbard RB , Card TR . BMJ (Clinical research ed.).2004;329(7468). CrossRef
- Deficient UDP-glucuronosyltransferase detoxification enzyme activity in the small intestinal mucosa of patients with coeliac disease Goerres M., Roelofs H. M. J., Jansen J. B. M. J., Peters W. H. M.. Alimentary Pharmacology & Therapeutics.2006;23(2). CrossRef
- Celiac disease autoimmunity is associated with leukocyte telomere shortening in older adults: The U.S. National Health and Nutrition Examination Survey Kamycheva E, Goto T, Camargo CA . Experimental Gerontology.2017;89. CrossRef
- High Risk of Digestive Cancers in Patients With Celiac Disease: A Nationwide Case-Control Cohort Study Jannot A, Girardeau Y, Chaussade S, Coriat R, Cerf-Bensussan N, Malamut G. Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association.2025;23(7). CrossRef
- Cancer Risk in 47,241 Individuals With Celiac Disease: A Nationwide Cohort Study Lebwohl B, Green PHR , Emilsson L, Mårild K, Söderling J, Roelstraete B, Ludvigsson JF . Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association.2022;20(2). CrossRef
- Intestinal and blood lymphograms as new diagnostic tests for celiac disease Roy G, Fernández-Bañares F, Corzo M, Gómez-Aguililla S, García-Hoz C, Núñez C. Frontiers in Immunology.2022;13. CrossRef
- Microbiome Markers in Gastrointestinal Disorders: Inflammatory Bowel Disease, Colorectal Cancer, and Celiac Disease San-Martin MI , Chamizo-Ampudia A, Sanchiz Á, Ferrero MA , Martínez-Blanco H, Rodríguez-Aparicio LB , Navasa N. International Journal of Molecular Sciences.2025;26(10). CrossRef
- HLA-DQ and risk gradient for celiac disease Megiorni F, Mora B, Bonamico M, Barbato M, Nenna R, Maiella G, Lulli P, Mazzilli MC . Human Immunology.2009;70(1). CrossRef
- The role of the gluten-free diet in the development of malignancies in celiac disease. Celiac Disease and Gluten-Free Diet Botosso M, Damasceno R, Farage P. 2023.
- Risk of malignancy in patients with celiac disease Green PH , Fleischauer AT , Bhagat G, Goyal R, Jabri B, Neugut AI . The American Journal of Medicine.2003;115(3). CrossRef
- Risk of Lymphoproliferative Malignancy in Relation to Small Intestinal Histopathology Among Patients With Celiac Disease Elfstrom P., Granath F., Ekstrom Smedby K., Montgomery S. M., Askling J., Ekbom A., Ludvigsson J. F.. JNCI Journal of the National Cancer Institute.2011;103(5). CrossRef
Content
Abstract Introduction Esophageal cancer (EC) Colorectal Cancer (CRC) Small Intestine Adenocarcinoma (SBA) Lymphoma Primary liver cell cancer/Hepatocellular Carcinoma (HCC) Breast Cancer (BC) Oropharyngeal Cancer (OPC) Endometrial Cancer Ovarian Cancer (OC) The main mechanisms of the association between celiac disease (CD) and cancer Relationship between celiac disease (CD) and cancers Diagnose types of cancers in patients with celiac disease (CD) The link between cancer and children with celiac disease (CD) Association between gluten-free diet (GFD) and various cancers Acknowledgments ReferencesLicense

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2026
Author Details