Hypoxia-Inducible Factor-1 Alpha Expression Correlates with Lymphovascular Invasion and Nodal Metastasis in Invasive Breast Cancer
Download
Abstract
Background: Breast cancer prognosis is critically influenced by lymphovascular invasion (LVI) and lymph node metastasis. Hypoxia-inducible factor-1 alpha (HIF-1α) is a key protein implicated in tumor progression and metastasis. This study aimed to investigate the relationship between HIF-1α expression and the presence of LVI and regional lymph node metastasis in invasive breast cancer.
Methods: This cross-sectional study analyzed 80 paraffin-embedded tissue specimens from participants with stage I–III invasive breast carcinoma. Participants were categorized based on the presence or absence of LVI and lymph node metastasis. HIF-1α expression was evaluated using immunohistochemistry and a semi-quantitative scoring system (IR-Score). The association between HIF-1α expression (positive vs. negative) and clinicopathological variables was analyzed using the Chi-Square test and odds ratio (OR) calculations.
Results: Positive HIF-1α expression was predominant in tumors with LVI (74.1 %) or lymph node metastasis (70.3 %). A significant association was found between HIF-1α expression and LVI (p=0.012), with HIF-1α-positive tumors showing an approximate four-fold increased risk for LVI (OR 3.896; 95 % CI 1.451–10.462) compared to HIF-1α-negative samples. Similarly, a significant association was observed between HIF-1α expression and lymph node metastasis (p=0.031), conferring an increased risk when compared to HIF-1α-negative tumors (OR 3.947; 95 % CI 1.256–12.409). The mean proportion of stained cells was significantly higher in LVI-positive tumors (p=0.015) and metastatic tumors (p=0.039).
Conclusion: HIF-1α expression is significantly associated with lymphovascular invasion and lymph node metastasis in invasive breast cancer. These findings underscore the potential of HIF-1α as a prognostic biomarker for tumors with high metastatic potential.
Introduction
Breast cancer is one of the most prevalent malignancies in women globally [1, 2]. Breast cancer has the highest incidence of all cancers in Indonesia, with approximately 42.1 cases per 100,000 women, and represents a major cause of cancer-related mortality at 16.6 deaths per 100,000 women in 2020 [1-4]. Tumor progression involves the spread of tumor cells (metastasis), which can occur lymphogenously or hematogenously [5]. Breast cancer prognosis is influenced by several factors, including histological grade, lymphovascular invasion (LVI), and lymph node metastatic status. The presence of tumor cell metastasis in regional lymph nodes dramatically worsens the prognosis, with the 5-year survival rate of these patients decreasing from over 90 % to as low as 20 % [6]. Hypoxia-inducible factor 1-alpha (HIF-1α) is a transcription factor that plays a crucial role in the cellular response to low oxygen levels and acts as a key mediator of tumor adaptation, promoting angiogenesis, metabolic reprogramming, and metastasis [7, 8]. Consequently, high levels of HIF-1α expression are often associated with poor prognosis and therapy resistance in breast cancer patients [7, 9, 10]. The potential of HIF-1α as a biomarker is relevant for patients with or without established metastases, as it plays a crucial role in the early stages of cancer development, including tumor initiation and progression [9, 11–14]. Therefore, assessing HIF-1α expression can provide valuable insights into tumor aggressiveness and potential therapeutic response, facilitating the development of more personalized and effective treatment strategies.
Beyond establishing lymph node status, LVI denotes a crucial early step in the metastatic cascade. Its presence in histopathological examination is a powerful independent prognostic factor that is strongly associated with an increased risk of regional lymph node metastasis, distant recurrence, and lower overall survival rate [15, 16]. The biological process of LVI involves the invasion of tumor cells into lymphatic or blood vessels, a process facilitated by new vessel formation (lymphangiogenesis) and increased cell motility, which are heavily influenced by the hypoxic tumor microenvironment, where HIF-1α is the master regulator [8, 9].
Given the critical role of LVI as a precursor to nodal metastasis and the established function of HIF-1α in promoting invasion and angiogenesis, a direct investigation into the relationship between these two components is essential. HIF-1α has been linked to metastasis; however, the association between HIF-1α expression and the presence of LVI in invasive breast cancer of no special type (NST) is unclear. To our knowledge, research exploring this specific relationship has not been conducted in the Makassar population. Therefore, this study aimed to determine the relationship between HIF-1α expression and the presence or absence of LVI or lymph nodes metastases in breast cancer patients in Makassar, Indonesia.
Materials and Methods
Study Design and Population
This cross-sectional study was conducted at Hasanuddin University Hospital, Dr. Wahidin Sudirohosodo General Hospital, and the Makassar Pathology Diagnostic Center Laboratory from April 2023 to August 2023. A minimum required sample size of 80 participants was calculated a priori using a standard formula for an unpaired categorical analytic study [17-19]. A consecutive sampling method was then employed. The study population comprised 80 paraffin blocks obtained from resections of mastectomies of the study participants. The method did not involve a random selection from a larger historical archive; rather, all patients meeting the inclusion criteria were enrolled sequentially until the pre-determined sample size of 80 participants was reached. All participants were diagnosed with stage I, II, or III invasive breast carcinoma and categorized based on the presence or absence of LVI and regional lymph node metastasis. Participants were selected according to predefined inclusion and exclusion criteria.
Inclusion and Exclusion Criteria
The inclusion criteria were: 1) Tissue from surgical mastectomy resections that was diagnosed by an anatomical pathologist as invasive breast carcinoma (Stage I, II, or III); 2) definitive assessment of LVI and regional lymph node status via Hematoxylin-Eosin (HE) staining; and 3) successful HIF-1α immunohistochemical staining and interpretation by two independent pathologists.
The exclusion criteria were: 1) Paraffin block preparations damaged during reprocessing; 2) patients diagnosed with Stage IV (metastatic) breast cancer; 3) cases of recurrent breast cancer; and 4) patients who had received neoadjuvant chemotherapy or radiotherapy prior to surgery.
Immunohistochemical Staining
Immunohistochemical staining was performed on tissue sections from the paraffin blocks using the standard avidin-biotin-peroxidase complex method. A polyclonal antibody against HIF-1α (Elabscience, Houston, TX, USA; Cat. No. E-AB-31662) was used. Staining results were evaluated under a light microscope by two pathologists.
Assessment of Variables
Lymphovascular Invasion (LVI): The presence of LVI was determined using HE-stained slides. A ‘positive’ classification was assessed if tumor cells were observed within endothelial-lined vascular or lymphatic channels; otherwise, a ‘negative’ designation was allocated.
Lymph Node Metastasis: The presence of metastasis was determined by the histopathological examination of axillary lymph nodes. The presence of tumor cells in the nodes indicated a ‘positive’ assessment, and the absence of these cells denoted a ‘negative’ result.
HIF-1α Expression: HIF-1α immunoexpression was evaluated using a semi-quantitative scoring system [20] involving the calculation of an Immunoreactive Score (IR-Score) by multiplying the staining intensity by the proportion of stained cells. This method was chosen as it provides a more comprehensive and objective measure of overall protein expression than the assessment of either parameter alone. An IR-Score of 0–2 was considered ‘negative’, while a score of 3–9 was considered ‘positive’.
Data Analysis
Data were processed using Microsoft Excel 2020 and SPSS version 26 (IBM Corp., Chicago, Ill., USA). The Chi-square test was used to assess the association between categorical variables (HIF-1α expression vs. LVI or metastasis status). The Mann-Whitney U test was used to compare the means of ordinal data (intensity, proportion, and IR-Score) between groups. A p-value of <0.05 was considered statistically significant.
Results
The characteristics of the 80 participants are summarized in Table 1.
| Variable | n (%) |
| Age (years) | |
| <50 | 37 (46.3) |
| ≥50 | 43 (53.8) |
| LVI | |
| Positive | 54 (67.5) |
| Negative | 26 (32.5) |
| Metastasis | |
| Positive | 64 (80.0) |
| Negative | 16 (20.0) |
| HIF-1α expression | |
| Positive | 51 (63.7) |
| Negative | 29 (36.3) |
The majority of participants were ≥50 years of age (53.8 %), with a mean age of 51.6 ± 10.4 years. High prevalences of lymph node metastasis and LVI presence were observed, with positive assessments in 64 (80.0 %) and 54 (67.5 %) participants, respectively. Based on immunohistochemical assessment, 51 participants (63.7 %) were classified as having positive HIF-1α expression. Positive HIF-1α expression was visualized as brown nuclear staining in tumor cells (Figure 1).
Figure 1. Immunohistochemical Staining of HIF-1α Expression Based on the Degree of Histopathology of Invasive Breast Carcinoma. HIF is observed in the nucleus of tumor cells. (Magnification 400x).
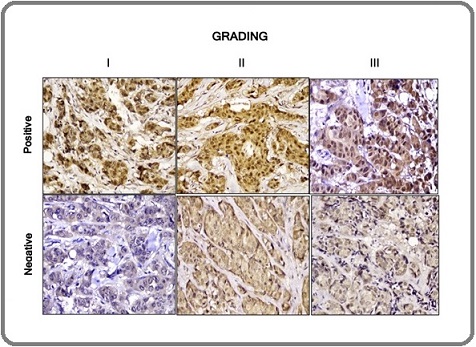
A significant association was identified between HIF-1α expression and LVI presence (p=0.012; Table 2).
| HIF-1α expression | LVI | OR (CI 95 %) | p-value * | |
| Positive | Negative | |||
| Positive | 40 (74.1) | 11 (42.3) | 3.896 | 0.012 |
| Negative | 14 (25.9) | 15 (57.7) | (1.451–10.462) |
Note, *Chi-square test; HIF-1α=hypoxia inducible factor-1 alpha; LVI = lymphovascular invasion
Tumors from participants with positive HIF-1α expression demonstrated an approximate four-fold higher risk of exhibiting lymphovascular invasion (OR 3.896, 95 % CI 1.451–10.462) compared to those with negative HIF-1α expression. Similarly, the majority of LVI-positive tumors (74.1 %) showed positive HIF-1α expression.
Table 3 shows a comparison of HIF-1α scoring components based on LVI status.
| HIF-1α expression | LVI (Mean±SD) | p-value * | |
| Positive | Negative | ||
| Intensity | 2.07±0.89 | 1.77±0.86 | 0.15 |
| Proportion | 2.26±0.76 | 1.81±0.75 | 0.015 |
| IR-Score | 4.93±2.96 | 3.58±2.82 | 0.036 |
Note, *Mann–Whitney test
While the mean staining intensity did not differ significantly between the LVI-positive and LVI-negative groups (p=0.150), the mean proportion of stained area was significantly higher in LVI-positive tumors (p=0.015). Consequently, the mean IR-Score was also significantly higher in the group with LVI (p=0.036).
Table 4 identifies a significant association (p=0.031) between HIF-1α expression and lymph node metastasis, indicating that HIF-1α-positive tumors had an approximately four-fold greater risk of metastasizing to regional lymph nodes (OR 3.947, 95 % CI 1.256–12.409) than HIF-1α-negative tumors.
| HIF-1α expression | Lymph node metastasis | OR (CI 95 %) | p-value * | |
| Positive | Negative | |||
| Positive | 45 (70.3) | 6 (37.5) | 3.947 | 0.031 |
| Negative | 19 (29.7) | 10 (62.5) | (1.256–12.409) |
Note, *Chi-square test, HIF-1α=hypoxia inducible factor-1 alpha; OR=odd ratio.
Correspondingly, 70.3 % of samples with positive lymph node metastasis showed positive HIF-1α expression.
When comparing scores based on metastatic status (Table 5), the mean proportion of stained area was significantly higher in tumors with lymph node metastases (p=0.039).
| HIF-1α expression | Metastasis (Mean±SD) | p-value * | |
| Positive | Negative | ||
| Intensity | 2.03±0.87 | 1.75±0.93 | 0.252 |
| Proportion | 2.20±0.76 | 1.75±0.77 | 0.039 |
| IR-Score | 4.73±2.92 | 3.50±3.01 | 0.077 |
Note, *Mann–Whitney test.
However, the differences in mean intensity (p=0.252) and the overall IR-Score (p=0.077) were not statistically significant between the metastatic and non- metastatic groups.
Discussion
The results of this study demonstrate a significant association between HIF-1α expression and two key indicators of tumor progression LVI and lymph node metastasis in invasive breast cancer. Our demographic finding that breast cancer was more prevalent in patients aged ≥50 years aligns with global epidemiology, which reports that cancer risk increases with age due to the accumulation of cellular modifications over time [21, 22].
Positive LVI findings were identified in the majority of the participants (67.5 %). The presence of LVI is a recognized histopathological feature of malignancy and metastatic potential, and in breast cancer, tumor emboli predominantly occur in lymphatic vessels. The reported frequency of LVI in breast cancer literature varies widely, from 8.8 % to 69.5 %; this fluctuation is often attributed to the time-intensive nature of LVI assessment and inter- observer variability. The rate observed in our study is consistent with the upper end of this reported range [23]. Furthermore, our study demonstrated that four times more participants showed lymph node metastasis compared to those without this condition. This result is consistent with the findings of Nathanson et al. (2022) [24], who reported that higher-grade invasive breast cancers tended to metastasize to lymph nodes. This process is associated with the role of the lymphatic system in the dissemination of breast cancer cells. As cancer cells develop, they can enter lymphatic vessels and spread to nearby lymph nodes, an event that is frequently associated with a poor prognosis [5, 24, 25].
Our results further reported that 51 of 80 participants (63.7 %) showed positive HIF-1α expression. HIF-1α is a dimeric protein complex that mediates the response of the body to hypoxic conditions. HIF-1α is not typically detected in normal human tissue due to its rapid degradation under normoxic conditions. However, its expression is markedly increased in several cancers, including breast, lung, and prostate cancer. Through its function in hypoxic environments, HIF-1α facilitates the adaptation of cancer cells by promoting angiogenesis to meet the increased demand for oxygen and nutrients, thereby contributing to tumor proliferation [26-28].
This study reported that the majority of participants with positive LVI also showed positive HIF-1α expression (74.1 %). This finding corroborates the research of Schito et al. (2012) [29], who demonstrated a linear correlation between HIF-1α, PDGF-B, and the area of LVI, suggesting that HIF-1α signaling is important for lymphangiogenesis and metastasis in breast cancer. Although our study did not measure mechanistic mediators directly, our statistical results suggest an indirect relationship between HIF- 1α expression and LVI. Vascular Endothelial Growth Factor-C (VEGF-C), a factor induced by HIF-1α, has been correlated with lymphangiogenesis in breast cancer, and Ni et al. (2013) [30] reinforced this association by showing that HIF-1α expression had a significant relationship with lymphatic microvessel density (LMVD) through its role in inducing VEGF-C. HIF-1α activates the transcription of several genes involved in neovascularization and tumor metastasis, including endothelin-1, VEGF, and inducible nitric oxide synthase [31].
Our study found no significant difference in mean staining intensity based on LVI status. Overall, while existing studies suggest that HIF-1α expression may enhance LVI in breast cancer through its effects on VEGF-C and PDGF-B expression, more research is needed to fully elucidate the direct relationship.
A significant relationship was found between HIF-1α expression and tumor cell metastasis to the lymph nodes; 70.3 % of participants with lymph node metastasis showed positive HIF-1α expression. This aligns with Liu et al. (2015) [32], who reported that high HIF-1α expression predicted metastasis and correlated with poor clinical outcomes in breast cancer. Those authors stated that HIF-1α regulates metastasis through various mechanisms, including mediating epithelial-mesenchymal transition (EMT), invasion, and metastatic niche formation. Similar studies support the role of HIF-1α expression in breast cancer metastasis, such as that of Schito et al. (2012) [29], who specified that HIF-1α plays an important role in lymphangiogenesis and lymphatic metastasis in breast cancer through its effect on Platelet-Derived Growth Factor B (PDGF-B) transcription [30-32].
Our study found no significant difference in mean intensity and IR-score between tumors that had metastasized to lymph nodes and those that had not. This observation appears to contrast with the established role of HIF-1α in promoting metastasis. However, this finding is consistent with the results of Kim and Simon (2022) [33], which indicated high HIF-1α expression in non-metastasized breast cancer tissue. The hypothesis of those authors suggests that while high HIF-1α expression may not universally initiate metastasis, metastasizing tumors with high HIF-1α levels show increased aggressiveness, resulting in a poorer prognosis for the patient.
One limitation of this study is that the analysis was confined to HIF-1α immunohistochemistry. Therefore, future research is warranted to correlate HIF-1α expression with the status of hormonal receptors (ER, PR, and Her-2) and the expression of related molecules such as VEGF-C, PDGF-B, endothelin-1, and nitric oxide synthase. In addition, while our study included a range of stages (I–III), we did not perform sub-analyses based on specific tumor burden characteristics (e.g., T-stage or N-stage), which could offer further insights into the relationship between tumor heterogeneity and HIF-1α expression. Finally, the use of a consecutive sampling method within a specific time frame can introduce temporal selection bias, as the patient cohort may not fully represent the characteristics of all patients diagnosed throughout the year. Future studies with longer enrollment periods could help validate the generalizability of these findings.
In conclusion, this study provides strong evidence that HIF-1α expression is significantly associated with lymphovascular invasion and lymph node metastasis in invasive breast cancer. These findings reinforce the role of HIF-1α as a critical driver of tumor progression and highlight its potential as a prognostic biomarker to identify patients at high risk of metastatic disease.
Acknowledgements
The researchers would like to express their sincere gratitude to the Faculty of Medicine at Hasanuddin University in Makassar, Indonesia, for their generous support of this research.
Contributors
ADC: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Project administration, Resources, Validation, Visualization, Writing – original draft, Writing – review & editing. BJN: Conceptualization, Data curation, Formal analysis, Funding acquisition, Investigation, Methodology, Data curation, Formal analysis, Investigation, Methodology, Project administration, Resources, Supervision, Software, Validation, Visualization, Writing – original draft. MHC: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Supervision, Validation, Visualization, Writing – original draft, Writing – review & editing. UAM: Conceptualization, Investigation, Methodology, Resources, Supervision, Software, Validation, Writing – original draft, Writing – review & editing. ST: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Validation, Writing – original draft, Writing – review & editing. GA: Conceptualization, Data curation, Formal analysis, Investigation, Methodology, Resources, Writing – original draft, Writing – review & editing.
Competing interests
No competing interests were reported.
Patient and public involvement
This research incorporated the perspectives of patients and/or members of the public throughout its design, conduct, reporting, and dissemination phases. Details are provided in the Methods section.
Ethics approval
The study was approved by the Research Ethics Committee of the Faculty of Medicine Universitas Hasanuddin, Makassar, Indonesia, number: 553/ UN4.6.4.5.31/PP.36/2023. We promised that the participants’ data were anonymized or maintained with confidentiality, the rights or interests of participants were not invaded, and informed consent was taken from all individual participants.
Data availability statement
Data is accessible upon justifiable request.
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- Centers for Disease Control and Prevention. What is breast cancer? Centers for Disease Control and Prevention 2022.
- Cancer Incidence and Mortality in a Tertiary Hospital in Indonesia: An 18-Year Data Review Prihantono , Rusli R, Christeven R, Faruk M. Ethiopian Journal of Health Sciences.2023;33(3). CrossRef
- Breast cancer incidence in Indonesia: a sex-disaggregated analysis using WHO health equity assessment toolkit data Osborne A, Adnani QES , Ahinkorah BO . BMC Cancer.2025;25(1). CrossRef
- Breast cancer metastasis and the lymphatic system Rahman M, Mohammed S. Oncology Letters.2015;10(3). CrossRef
- Invasive Breast Carcinoma of No Special Type. In: Lokuhetty D, ed. WHO Classification of Tumours: Breast Tumours, 5th ed. France Rakha E, Allison K, Schnitt S, Llorca FP , Bu H. International Agency for Pesearch on Cancer (lABC).2019;102(109):102-109.
- The role of hypoxia-inducible factor-1 alpha in multidrug-resistant breast cancer Yong L., Tang S., Yu H., Zhang H., Zhang Y., Wan Y.. Front Oncol.2022;12. CrossRef
- Expression of HIF-1α and markers of angiogenesis and metabolic adaptation in molecular subtypes of breast cancer Farooq M., GhR B, Besina S., Thakur N., Zahoor S., Rather R.A.. Transl Med Commun.2023;8(2). CrossRef
- Hypoxia inducible factor-1α (HIF-1α) in breast cancer: The crosstalk with oncogenic and onco-suppressor factors in regulation of cancer hallmarks Mirzaei S., Ranjbar B., Tackallou S.H., Aref A.R.. Pathol Res Pract.2023;248(154676). CrossRef
- Extracellular ATP promotes breast cancer chemoresistance via HIF-1α signaling Yang H., Geng Y.-H., Wang P., Zhang H.-Q., Fang W.-G., Tian X.-X.. Cell Death Dis.2022;13(199). CrossRef
- A novel HIF-2α targeted inhibitor suppresses hypoxia-induced breast cancer stemness via SOD2-mtROS-PDI/GPR78-UPRER axis Yan Y., He M., Zhao L., Wu H., Zhao Y., Han L.. Cell Death Differ.2022;29. CrossRef
- Hypoxia-Inducible Factors-Based Single Nucleotide Polymorphism in Breast Cancer with More Cancer Susceptibility Ray S.K., Mukherjee S.. Curr Mol Med.2023;23. CrossRef
- Hypoxia-inducible factor in breast cancer: role and target for breast cancer treatment Zhi S., Chen C., Huang H., Zhang Z., Zeng F., Zhang S.. Front Immunol.2024;15. CrossRef
- Targeting hypoxia-inducible factors for breast cancer therapy: A narrative review Luo S., Jiang Y., Zheng A, Zhao Y., Wu X., Li M.. Front Pharmacol.2022;13. CrossRef
- Association between lymphovascular invasion (LVI) and prognostic factors in breast cancer Kabir F., Tani S.A., Ullah M.A., Hassan A.F.U., Islam M.J., Ansary J.. Journal of Shaheed Suhrawardy Medical College.2024;14. CrossRef
- Lymphovascular invasion is an independent prognostic factor in breast cancer irrespective of axillary node metastasis and molecular subtypes Lee SJ , Go J, Ahn BS , Ahn JH , Kim JY , Park HS , Kim SI , Park B, Park S. Frontiers in Oncology.2023;13. CrossRef
- Biostatistics Series Module 4: Comparing Groups – Categorical Variables Hazra A, Gogtay N. Indian Journal of Dermatology.2016;61(4). CrossRef
- Basic statistical tools in research and data analysis Ali Z, Bhaskar S. Indian Journal of Anaesthesia.2016;60(9). CrossRef
- Factors associated with Leptospira Serodiagnosis in febrile patients at public Health Centers in Makassar, Indonesia: a cross-sectional study Cahyaningtyas C, Muslich LT , Madjid B, Sultan AR , Hamid F, Hatta M. Pan African Medical Journal.2024;49. CrossRef
- Strong Expression of Hypoxia-Inducible Factor-1α (HIF-1α) Is Associated with Axl Expression and Features of Aggressive Tumors in African Breast Cancer Nalwoga H, Ahmed L, Arnes JB , Wabinga H, Akslen LA . PLOS ONE.2016;11(1). CrossRef
- Breast Cancer—Epidemiology, Risk Factors, Classification, Prognostic Markers, and Current Treatment Strategies—An Updated Review Łukasiewicz S, Czeczelewski M, Forma A, Baj J, Sitarz R, Stanisławek A. Cancers.2021;13(17). CrossRef
- The Etiology of Breast Cancer Twort C.C., Bottomley A.C.. The Lancet.1932;220(5693). CrossRef
- Updates on Lymphovascular Invasion in Breast Cancer Kuhn E, Gambini D, Despini L, Asnaghi D, Runza L, Ferrero S. Biomedicines.2023;11(3). CrossRef
- Mechanisms of breast cancer metastasis Nathanson SD , Detmar M, Padera TP , Yates LR , Welch DR , Beadnell TC , Scheid AD , Wrenn ED , Cheung K. Clinical & Experimental Metastasis.2022;39(1). CrossRef
- Prediction of Sentinel Lymph Node Metastasis Using the Platelet-to-lymphocyte Ratio in T1 Breast Cancer Takada K, Kashiwagi S, Asano Y, Goto W, Kouhashi R, Yabumoto A, Morisaki T, et al . Anticancer Research.2020;40(4). CrossRef
- Hypoxia-Inducible Factor (HIF)-1 regulatory pathway and its potential for therapeutic intervention in malignancy and ischemia Ziello JE , Jovin IS , Huang Y. The Yale Journal of Biology and Medicine.2007;80(2).
- The Role of Hypoxia-Inducible Factor Isoforms in Breast Cancer and Perspectives on Their Inhibition in Therapy Kozal K., Krześlak A.. Cancers (Basel.2022;14. CrossRef
- Hypoxia-inducible factor-1α expression and breast cancer recurrence in a Danish population-based case control study Collin L.J., Maliniak M.L., Cronin-Fenton D.P., Ahern T.P., Christensen K.B., Ulrichsen S.P.. Breast Cancer Research.2021;23. CrossRef
- Hypoxia-inducible factor 1-dependent expression of platelet-derived growth factor B promotes lymphatic metastasis of hypoxic breast cancer cells Schito L., Rey S., Tafani M., Zhang H., Wong C.C.L., Russo A.. Proc Natl Acad Sci U S A.2012;109. CrossRef
- Hypoxia-induced factor-1 alpha upregulates vascular endothelial growth factor C to promote lymphangiogenesis and angiogenesis in breast cancer patients Ni X., Zhao Y., Ma J., Xia T., Liu X., Ding Q.. J Biomed Res.2013;27. CrossRef
- Expression and significance of PTEN, hypoxia-inducible factor-1 alpha in colorectal adenoma and adenocarcinoma Jiang Y.-A., Fan L.-F., Jiang C.-Q., Zhang Y.-Y., Luo H.-S., Tang Z.-J.. World J Gastroenterol.2003;9. CrossRef
- Hypoxia-inducible factor 1 and breast cancer metastasis Liu Z. ji , Semenza GL , Zhang H. feng . J Zhejiang Univ Sci B.2015;16. CrossRef
- Hypoxia-Inducible Factors in Cancer Kim L.C., Simon M.C.. Cancer Res.2022;82. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2026
Author Details