Co-expression of BCL2 and C-MYC as a Potential Predictive Factor of Survival in Diffuse Large B Cell Lymphoma with Rituximab-Based Chemotherapy
Download
Abstract
Objective: Diffuse Large B Cell Lymphoma (DLBCL) is a heterogeneous disease with varying aggressiveness and response to therapy. The co-expression of BCL2 and C-MYC has been reported to hold prognostic value for disease outcomes. This study aimed to determine the proportion of DLBCL patients with co-expression of BCL2 and C-MYC at Dr. Sardjito Hospital and evaluate its association with clinicopathological features and survival.
Methods: This retrospective cohort study included 72 DLBCL patients treated with rituximab-based chemotherapy between January 2017 and December 2019. The co-expression of BCL2 and C-MYC was evaluated using immunohistochemistry. The Kaplan-Meier method was used along with the log-rank test to assess 5 years overall survival and to compare the two groups. Cox proportional hazards regression was applied to identify factors affecting survival.
Result: The proportion of patients with BCL2 and C-MYC co-expression was 12.5% (9/72 patients). These patients exhibited a trend toward poorer survival compared to those without co-expression (median OS: 26 months [95% CI: 0.000–52.296] vs. 71 months [95% CI: 55.057–86.943]; p = 0.156). However, the co-expression of BCL2 and C-MYC did not demonstrate predictive value for 5-year survival in DLBCL patients receiving rituximab-based chemotherapy.
Conclusion: DLBCL patients with BCL2 and C-MYC coexpression demonstrated a trend toward shorter survival compared to those without co-expression. However, this co-expression was not identified as an independent prognostic factor for 5-year survival in DLBCL patients treated with rituximab-based chemotherapy.
Introduction
Diffuse Large B Cell lymphoma (DLBCL) is the most common type of B cell Non Hodgkin’s Lymphoma (NHL), accounting for approximately 30%-40% of all B cell NHL cases. The disease heterogeneity affects its aggressiveness and response to therapy [1]. The introduction of rituximab, an anti-CD20 monoclonal antibody that specifically targets B-cells, has significantly improved outcomes for DLBCL patients. Progression-free survival (PFS) rates have increased from 51% to 69% in the rituximab era [2]. Despite this advancement, the response rate to initial therapy remains at 40%–60%, and the relapse rate can reach up to 75% within five years, highlighting the ongoing challenge in achieving durable remission [1]. These has prompted research into prognostic and predictive factors of DLBCL.
Previous studies have investigated a range of biomarkers associated with treatment outcomes, including clinical variables (such as age and International Prognostic Index (IPI) score), molecular charactheristics such as cell-of-origin (COO), chromosomal rearrangements, and various protein expressions profiles [3]. Among these, the co-expression of BCL2 and C-MYC proteins, known as Double-Expressor Lymphoma (DEL), has been reported as a marker of poor prognosis and treatment resistance when assessed using immunohistochemistry [4]. Therefore, this study aimed to determine the proportion of DEL cases among DLBCL patients treated at Dr Sardjito Hospital, a tertiary referral center in Yogyakarta, Indonesia, and to evaluate the association between DEL status, clinicopathological features and overall survival (OS).
Materials and Methods
Patients and Data Collection
This study employed a retrospective design, utilizing data extracted from the clinical registry of lymphoma patients who were diagnosed and treated at Sardjito General Hospital in the Division of Hematology and Medical Oncology, Department of Internal Medicine, between January 2017 to December 2019. The data included sociodemographic characteristics, clinical profiles, and overall survival. Data extraction commenced after ethical clearance was obtained. The study protocol was reviewed and approved by the Ethical Committee of the Faculty of Medicine, Universitas Gadjah Mada (Approval Number: KE/1516/10/2023).
Immunohistochemistry Analysis
Immunohistochemical examinations for BCL2 and C-MYC were performed on DLBCL biopsy specimens in paraffin blocks from the laboratory of Anatomical Pathology of Dr Sardjito Hospital. Tissue samples from patients with DLBCL were obtained in compliance with the recommendations of the Declaration of Helsinki for Biomedical Research. Informed consent was obtained from subjects involved in the study. Pathological examination was performed under an OLYMPUS CX33 microscope using Sigma’s Optilab camera and Optilabviewer4 application. BCL2 protein expression indicated by brown granules in the cytoplasm, while C-MYC protein appeared as brown coloured granules in the cell nucleus. BCL2 and C-MYC expression values were presented as percentages determined from the ratio of total positive tumour cells in 100 tumour cells. A positive value for C-MYC is more than >40% and BCL2 is more than >50% [5, 6]. Two pathological anatomy experts performed Immunohistochemistry staining results for BCL2 and C-MYC examination. The interrater agreement by Cohen’s Kappa was 0.972.
Statistical Consideration
The data were presented in tables and figures. Comparisons between variables were analysed by t-test with statistical significance set at p<0.05. Univariate analysis was used to evaluate the association of each factor with overall survival. Survival analysis utilized Kaplan-Meier method, and differences between survival curves were assessed with the log-rank test. To identify independent prognostic factors while adjusting for other variables, multivariate analysis was carried out using the Cox proportional hazards regression model.
Results
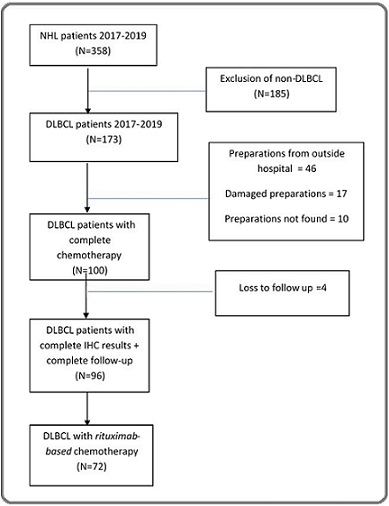
A total of 358 cases of non-Hodgkin’s lymphoma (NHL) were recorded between January 2017 to December 2019, consisting of 173 (48.3%) cases of DLBCL and 185 (51.7%) of non-DLBCL. Seventy-three (73) cases were excluded due to the following reasons: 46 cases had anatomical pathology preparations originating from outside Dr. Sardjito General Hospital, 17 cases had damaged paraffin blocks that could not be stained, and 10 cases had missing preparations. Based on the available clinical data and the presence of optimal and traceable paraffin blocks, 100 DLBCL cases out of 173 meeting the inclusion criteria were selected as research subjects. These cases fulfilled the requirements for high-quality paraffin block preparations and successful immunohistochemical staining for BCL2 and C-MYC. Of these 100 DLBCL patients, only 72 received rituximab-based chemotherapy and were included in the final survival analysis. Figure 1 illustrates the flowchart of the study population.
Figure 1. Flowchart of the Study Population.
Based on the basic characteristics, the majority of patients were male (55.6%), while females were 44.4%. Patients aged ≥60 years accounted for 54.2%, whereas those aged <60 years accounted for 45.8%. Most DLBCL patients were classified as Ann Arbor stage III-IV (52.7%), while 47.3% were in the stage I-II. The majority had an ECOG performance status <2 (94.4%) and extranodal involvement of ≤2 sites (98.6%). Among the patients, 39 (54.2%) were positive for BCL-2 expression, while 33 (45.8%) were negative. C-MYC expression was positive in 14 patients (19.4%) and negative in 58 patients (80.6%).
Co-expression of BCL-2 and C-MYC was identified in 9 patients (12.5%), whereas 63 patients (87.5%) had no co-expression. These findings are summarized in Table 1, with comparisons of clinicopathological features between the two groups are further shown in Table 2.
| Characteristics | N (72) | % | |
| Age, median (range) | 62 (27-82) | ||
| <60 years old | 33 | 45.83 | |
| ≥60 years old | 39 | 54.16 | |
| Gender | male | 40 | 55.56 |
| female | 32 | 44.45 | |
| Stage | stage I-II | 34 | 47.23 |
| stage III-IV | 38 | 52.73 | |
| ECOG a | <2 | 68 | 94.45 |
| ≥2 | 4 | 5.56 | |
| Extranodal involvement | ≤2 | 71 | 98.61 |
| >2 | 1 | 1.38 | |
| BCL2 | negative | 33 | 45.83 |
| positive | 39 | 54.16 | |
| C-MYC | negative | 58 | 80.55 |
| positive | 14 | 19.44 | |
| BCL2/CMYC | no | 63 | 87.5 |
| co-expression | yes | 9 | 12.5 |
| Variables (N=72) | BCL2/CMYC co-expression | Odds Ratio (CI 95%) | p | |||||
| No (63) | Yes (9) | |||||||
| n | % | n | % | |||||
| Age | <60 years old | 30 | 47.6 | 3 | 33.3 | 1.818 (0.417-7.919) | 0.331 ** | |
| ≥60 years old | 33 | 52.4 | 6 | 66.7 | ||||
| Ann-Arbor Stadium | Stage I-II | 36 | 57.1 | 5 | 55.6 | 1.067 (0.261-4.353) | 0.601 ** | |
| Stage III-IV | 27 | 42.9 | 4 | 44.4 | ||||
| ECOG a | <2 | 61 | 96.8 | 7 | 77.8 | 8.714 (1.056-71.896) | 0.074 ** | |
| ≥2 | 2 | 3.2 | 2 | 22.2 | ||||
| Extranodal involvement | ≤2 | 62 | 98.4 | 9 | 100 | 0.873 (0.799-0.954) | 0.874 ** | |
| >2 | 1 | 1.6 | 0 | 0 |
Notes: *significant at p<0.05.a: Eastern Cooperative Oncology Group. Odds ratio.* chi-square test, **fischer exact
Table 2 shows that age, stage, and extranodal involvement were not significantly associated with BCL2 and C-MYC co-expression. Patients aged ≥60 years were more frequent in the co-expression group compared to the non-co-expression group (66.7% vs. 52.4%). Similarly, stage III-IV was slightly more common in the co-expression group (44.4% vs. 42.9%). The ECOG performance status approached significance (p = 0.074), with ECOG ≥2 being more common in the co-expression group compared to the non-co-expression group (22.2% vs. 3.2%).
The mean follow-up time was 44.22 months, with the median follow-up of 52.5 months. Overall survival analysis using the Kaplan-Meier method for DLBCL patients who received rituximab-based chemotherapy is presented in Figure 2 .
Figure 2. Estimated 5-year Survival of DLBCL Patients Receiving Rituximab-based Chemotherapy.
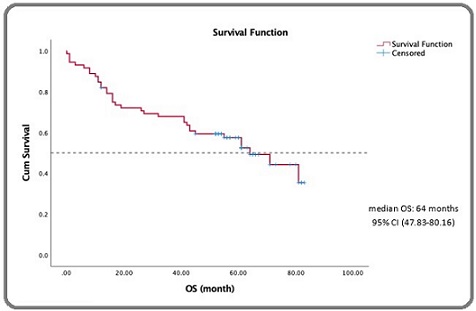
The median 5-year survival was 64 months (95% CI: 47.91–80.09 months), the mean survival was 53.79 months (95% CI: 46.37–62.21 months). Based on the curve, the 5-year survival rate was 51.4%, and the 3-year survival rate was 67%.
The 5-year OS of DLBCL patients who received rituximab-based chemotherapy when stratified by age, as shown in Figure 3, showed that patients aged <60 years had not reached the median survival value, whereas patients aged ≥ 60 years had a median survival of 45 months (95% CI: 28.90–61.09 months).
Figure 3. Estimated 5-year Survival of DLBCL Patients Receiving Rituximab-based Chemotherapy Stratified by Age.
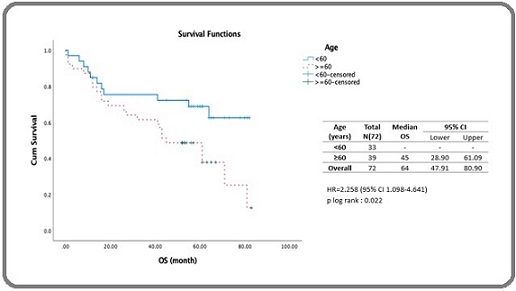
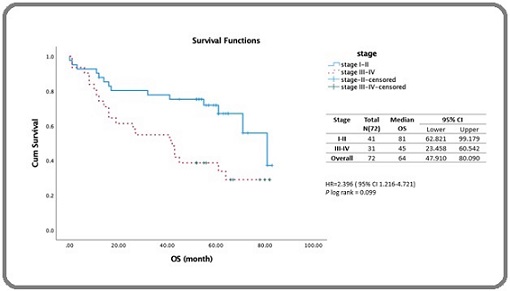
A log-rank test yielded a p-value of 0.022, indicating a significant difference in median survival between the two age groups. From the Cox proportional hazards model analysis, the hazard ratio (HR) for patients aged ≥ 60 years was 2.258 (95% CI: 1.098–4.641) with a p-value of 0.027 (Table 3). As shown in Figure 4, patients with stage III–IV disease had significantly lower survival than those with stage I–II disease : 42 months (95% CI: 23.45–60.54 months) vs. 81 months (95% CI: 62.81–99.17 months), with a log-rank p-value of 0.009, indicating a significant difference between the two groups. Cox proportional hazards yielded an HR of 2.396 (95% CI: 1.216-4.721) with a p-value of 0.012 (Table 3).
Figure 4. Estimated 5-year Survival of DLBCL Patients Receiving Rituximab-based Therapy Stratified by Stage.
| Variables | Bivariate | Multivariate | ||||||||
| HR | 95% CI | P | HR | 95% CI | P | |||||
| under | above | under | above | |||||||
| Gender (male vs female) | 0.955 | 0.487 | 1.872 | 0.892 | - | |||||
| Age (<60 vs ≥60) | 2.258 | 1.098 | 4.641 | 0.027 * | 2.106 | 0.994 | 4.459 | 0.052 | ||
| Stage ((1-II) vs (III-IV)) | 2.396 | 1.216 | 4.721 | 0.012 * | 2.572 | 1.29 | 5.127 | 0.007 | ||
| ECOG (<2 vs ≥2 ) | 4.73 | 1.419 | 15.769 | 0.011 * | 4.36 | 1.241 | 15.314 | 0.022 | ||
| Extranodal (≤ 2 vs >2) | 6.631 | 0.849 | 51.811 | 0.071 | - | |||||
| BCL 2 (-vs+) | 1.131 | 0.577 | 2.215 | 0.719 | - | |||||
| CMYC (-vs+) | 1.587 | 0.718 | 3.507 | 0.254 | - | |||||
| BCL2/CMYC co-expression (no vs yes) | 1.865 | 0.722 | 4.508 | 0.166 | - |
*Analysed with Cox proportional hazard model significant at p<0.05
Analysis by ECOG performance status (Figure 5) showed a stark contrast in outcomes that patients with an ECOG performance status ≥2 had a significantly lower median survival compared to those with an ECOG <2.
Figure 5. Estimated 5-year Survival of DLBCL Patients Receiving Rituximab-based Chemotherapy Stratified by ECOG.
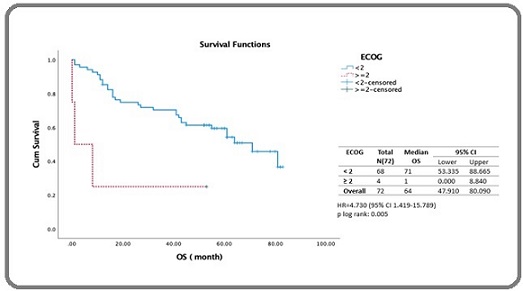
The median survival for the ECOG ≥ 2 group was 1 month (95% CI: 0.00–8.84 months), while for the ECOG <2 group, it was 71 months (95% CI: 53.55–88.66 months). The log-rank test showed a highly significant difference (p= 0.005) (Figure 5). Cox regression revealed an HR of 4.730 (95% CI: 1.419–15.789, p = 0.011), indicating ECOG ≥2 was strongly associated with poorer survival (Table 3).
The survival analysis stratified by BCL2 and C-MYC co-expression, showed that the median survival for the BCL2 and C-MYC co-expression group was 26 months (95% CI: 0.00–52.96 months), compared to 71 months (95% CI: 55.05–86.94 months) for the non-co-expression group, although the difference was not statistically significant (log rank p=0.156) (Figure 6).
Figure 6. Estimated 5-year Survival of DLBCL Patients Receiving Rituximab-based Therapy Stratified by Co-expression of BCL-2 and C-MYC.
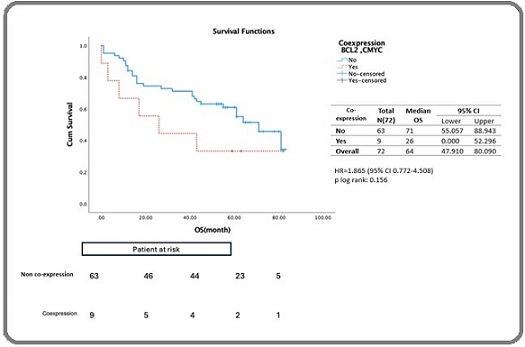
Cox proportional hazards produced an HR of 1.865 (95% CI: 0.772–4.508, p= 0.166) (Table 3), indicating no independent association with OS. Age, stage, and ECOG were significant factors influencing survival, with stage and ECOG identified as independent factors affecting survival as shown in Table 3.
Discussion
The prevalence of co-expression of BCL2 and C-MYC in this study was 12.5%, which is comparable to the results of a study conducted in Malaysia in 2019, where the prevalence was 13.3% [7]. Other studies have reported higher co-expression rates ranging from 20% to 35% in DLBCL [4, 8, 9]. The variations in co-expression rates between Asian and East Asian populations compared to European populations is likely due to differences in the proportion of patients with C-MYC expression reported in each study. Populations with a higher proportion of patients expressing C-MYC of >50% tend to have higher rates of BCL2 and C-MYC co-expression [10].
In this study, the proportion of BCL2 and C-MYC co-expression patients aged 60 years and older was higher than those aged under 60 years(66.7% vs. 52.4%). This finding is consistent with previous studies showing that BCL2 and C-MYC co-expression is more common among older patients, with one study reporting a prevalence of 56.2% in older patients versus 30.3% in younger patients [7]. Furthermore, co-expression was associated with poorer performance status, with ECOG ≥2 present in 22.2% of co-expression cases, compared to 3.2% in the non-co-expression group. This is in line with the results of studies that reported that patients with BCL2 and C-MYC co-expression generally had worse ECOG [11, 12]. The proportion of patients with BCL2 and C-MYC co-expression was higher in stages III–IV compared to the non-co-expression group. This aligns with previous studies reporting that co-expression of BCL2 and C-MYC is more prevalent in advanced Ann Arbor stages [10, 12]. In contrast, extranodal involvement was not significantly associated with BCL2 and C-MYC co-expression in our study. All patients in the co-expression group had involvement of two or less extranodal sites. This finding differs from studies reporting that co-expression is linked to increased extranodal involvement [13]. This discrepancy may be attributed to the small number of patients with extranodal involvement in our cohort, which could be related to the variability in staging modalities (e.g., X-ray, ultrasound, and CT scan), and the absence of PET scans at the study site.
Patients with BCL2 and C-MYC co-expression in this study tended to have shorter median survival compared to the non-co-expression group, although the difference was not statistically significant. This trend is consistent with reports from a meta-analysis, which showed a significant association between BCL2 and C-MYC co-expression and worse survival, with a pooled HR of 2.58 (95% CI: 1.19–3.04) [4, 14-16]. In our study, patients with co-expression were also at higher risk of death, in line with reports that identify co-expression as a marker of poor prognosis in DLBCL [15, 16]. Furthermore, co-expression of BCL2 and C-MYC is associated with aggressive disease, a tendency to occur in older age groups, increased extranodal involvement, advanced stages, and poor response to standard therapy namely rituximab-based chemotherapy [14, 17]. Previous studies have demonstrated that rituximab improves survival in patients without co-expression, but offers limited benefit in those with BCL2 and C-MYC co-expression [4] [10]. A retrospective study comparing R-CHOP and R-EPOCH regimens found a significantly higher recurrence rate in patients treated with R-CHOP (80%) compared to R-EPOCH (18%) [18]. These findings suggest that R-CHOP may be inadequate for this subgroup, although evidence on optimal alternative regimens remains limited.
Several studies have reported that the co-expression of BCL2 and C-MYC is a negative predictor of survival [3, 17]. The mechanism responsible for this is that co-expression creates a synergistic effect, with C-MYC stimulating cell proliferation and BCL2 inhibiting apoptosis, leading to increasingly uncontrolled lymphoma cell proliferation [15].
From the multivariate analysis, stage and ECOG remained significant, indicating that these are independent predictive factors affecting survival time in this cohort receiving rituximab-based therapy, while BCL2 and C-MYC co-expression was not. Several factors may explain this finding, 1) The proportion of patients with BCL2 and C-MYC co-expression in this study was smaller compared to the global population (12.5% vs. 20–30%), limiting statistical power to detect significance,2). The heterogeneity of DLBCL encompassing a wide spectrum of molecular and genetic subtypes, where co-expression of BCL2 and C-MYC represents only a subset of these variations, and other alterations (e.g., TP53 mutations or other unmeasured biomarkers) may have a stronger impact on survival [17], 3) Other co-existing prognostic factors: patients with BCL2 and C-MYC co- expression often also have high IPI scores or other adverse clinical features, which may overshadow the prognostic impact of co-expression alone, making it appear non- significant as independent predictive factor [19].
In conclusion, BCL2 and C-MYC co-expression was associated with a tendency toward poorer survival outcomes. However, it did not emerge as an independent prognostic factor for 5-year survival in DLBCL patients receiving rituximab-based chemotherapy, highlighting the need for larger studies to further clarify its clinical significance.
Acknowledgements
Funding Statement: This research was funded by the Final Project Recognition Program, Batch 1, 2022, through the Directorate of Research, Universitas Gadjah Mada.
Conflict of Interest
The authors declare no conflict of interest.
Ethical Declaration
Tissue samples from patients with DLBCL tumors were obtained in compliance with the recommendations of the Declaration of Helsinki for Biomedical Research. Informed consent was obtained from subjects involved in the study. The ethical clearance for this study was approved by the Ethical Committee of Faculty of Medicine, Universitas Gadjah Mada with number KE/1516/10/2023.
Authors Contribution
KS contributed for Data curation, Formal analysis, Investigation, Methodology, Resources, Software, Validation, Visualization and Writing of the original draft. MSH was responsible for Conceptualization, Funding acquisition, Investigation, Methodology, Resources, Supervision, Validation, Writing-original draft, Writing-review and editing. NA contributed for the Formal analysis, Investigation, Validation, Visualization, Writing-review and editing. KW was in charge for the Data curation, Formal analysis, Investigation, Methodology, Software, Supervision, Validation, Visualization, Writing-review and editing.
References
- Diffuse Large B-Cell Lymphoma: Recognition of Markers for Targeted Therapy Tomas-Roca L, Rodriguez M, Alonso-Alonso R, Rodriguez-Pinilla SM , Piris MA . Hemato.2021;2(2). CrossRef
- Double hit diffuse large B-cell lymphomas: diagnostic and therapeutic challenges Friedberg JW . Chinese Clinical Oncology.2015;4(1). CrossRef
- Double hit and double expressors in lymphoma: Definition and treatment Riedell PA , Smith SM . Cancer.2018;124(24). CrossRef
- Concurrent Expression of MYC and BCL2 in Diffuse Large B-Cell Lymphoma Treated With Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Johnson NA , Slack GW , Savage KJ , Connors JM , Ben-Neriah S, Rogic S, Scott DW , et al . Journal of Clinical Oncology.2012;30(28). CrossRef
- The 2016 revision of the World Health Organization classification of lymphoid neoplasms Swerdlow SH , Campo E, Pileri SA , Harris NL , Stein H, Siebert R, Advani R, et al . Blood.2016;127(20). CrossRef
- The Incidence and Treatment Response of Double Expression of MYC and BCL2 in Patients with Diffuse Large B-Cell Lymphoma: A Systematic Review and Meta-Analysis Hwang J, Suh C, Kim K, Kim H, Kim A, Craig J, Chen K, et al . Cancers.2021;13(13). CrossRef
- Clinical Significance of BCL2, C-MYC, and BCL6 Genetic Abnormalities, Epstein-Barr Virus Infection, CD5 Protein Expression, Germinal Center B Cell/Non-Germinal Center B-Cell Subtypes, Co-expression of MYC/BCL2 Proteins and Co-expression of MYC/BCL2/BCL6 Proteins in Diffuse Large B-Cell Lymphoma: A Clinical and Pathological Correlation Study of 120 Patients Ting CY , Chang KM , Kuan JW , Sathar J, Chew LP , Wong OLJ , Yusuf Y, et al . International Journal of Medical Sciences.2019;16(4). CrossRef
- MYC Alterations in Diffuse Large B-Cell Lymphomas Karube K, Campo E. Seminars in Hematology.2015;52(2). CrossRef
- The Role of c-MYC in B-Cell Lymphomas: Diagnostic and Molecular Aspects Nguyen L, Papenhausen P, Shao H. Genes.2017;8(4). CrossRef
- Molecular Profiling of High-Grade B-Cell Non-Hodgkin Lymphomas and Its Clinicopathologic Correlation in an Indian Tertiary Cancer Care Center Sebastian N, P. N SN , Ananthkrishnan R, Kumanan J., Chandran C, Raja T. Indian Journal of Medical and Paediatric Oncology.2024;45(06). CrossRef
- MYC/BCL2 protein coexpression contributes to the inferior survival of activated B-cell subtype of diffuse large B-cell lymphoma and demonstrates high-risk gene expression signatures: a report from The International DLBCL Rituximab-CHOP Consortium Program Hu S, Xu-Monette ZY , Tzankov A, Green T, Wu L, Balasubramanyam A, Liu WM , et al . Blood.2013;121(20). CrossRef
- Beyond RCHOP: A Blueprint for Diffuse Large B Cell Lymphoma Research Nowakowski GS , Blum KA , Kahl BS , Friedberg JW , Baizer L, Little RF , Maloney DG , et al . Journal of the National Cancer Institute.2016;108(12). CrossRef
- The significance of concurrent MYC and BCL2 expression in Egyptian patients with diffuse large B-cell NHL Abdelhamid TM , Gaber AA , Abdelfattah RM , Algamal DA , Hamdy O, Mohamed G. Pathology - Research and Practice.2024;253. CrossRef
- Immunohistochemical Double-Hit Score Is a Strong Predictor of Outcome in Patients With Diffuse Large B-Cell Lymphoma Treated With Rituximab Plus Cyclophosphamide, Doxorubicin, Vincristine, and Prednisone Green TM , Young KH , Visco C, Xu-Monette ZY , Orazi A, Go RS , Nielsen O, et al . Journal of Clinical Oncology.2012;30(28). CrossRef
- MYC and BCL2 protein expression predicts survival in patients with diffuse large B-cell lymphoma treated with rituximab Perry AM , Alvarado‐Bernal Y, Laurini JA , Smith LM , Slack GW , Tan KL , Sehn LH , et al . British Journal of Haematology.2014;165(3). CrossRef
- Prognostic significances of overexpression MYC and/or BCL2 in R-CHOP-treated diffuse large B-cell lymphoma: A Systematic review and meta-analysis Li L, Li Y, Que X, Gao X, Gao Q, Yu M, Ma K, Xi Y, Wang T. Scientific Reports.2018;8(1). CrossRef
- Double-Expressor Phenotype (BCL-2/c-MYC Co-expression) of Diffuse Large B-Cell Lymphoma and Its Clinicopathological Correlation Hashmi AA , Iftikhar SN , Nargus G, Ahmed O, Asghar IA , Shirazi UA , Afzal A, Irfan M, Ali J. Cureus.2021. CrossRef
- Outcome of Patients with Double-Expressor Lymphomas (DELs) Treated with R-CHOP or R-EPOCH Aggarwal A, Rafei H, Alakeel F, Finianos AN , Liu ML , El-Bahesh E, Ascensao JL , Mobarek D. Blood.2016;128(22). CrossRef
- MYC gene rearrangements are associated with a poor prognosis in diffuse large B-cell lymphoma patients treated with R-CHOP chemotherapy Savage KJ , Johnson NA , Ben-Neriah S, Connors JM , Sehn LH , Farinha P, Horsman DE , Gascoyne RD . Blood.2009;114(17). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2026
Author Details