Prognostic Significance of Tumor-Stroma Ratio in Hepatocellular and Gallbladder Carcinomas: A Systematic Review and Meta-Analysis
Download
Abstract
Background: The tumor microenvironment plays a crucial role in cancer progression, with the tumor-stroma ratio (TSR) emerging as a prognostic marker in solid tumors. A high TSR, indicating a greater proportion of tumor cells relative to stromal tissue, has been associated with improved survival. However, its prognostic value in hepatocellular carcinoma (HCC) and gallbladder carcinoma (GBC) remains unclear. This meta-analysis aims to assess the prognostic significance of TSR in these cancers.
Materials and methods: A systematic search of PubMed, Scopus, and Web of Science was conducted following PRISMA guidelines. Eligible cohort studies assessing TSR in HCC and GBC were included. Hazard ratios (HRs) for overall survival were pooled using a random-effects model. Heterogeneity was assessed using the I² statistic. The risk of bias was evaluated using the Newcastle-Ottawa Scale.
Results: Four retrospective cohort studies with 542 patients were included. A high TSR was significantly associated with improved survival in HCC (HR: 2.566, 95% CI: 1.028–4.104) but showed a weaker, non-significant association in GBC (HR: 1.568, 95% CI: 0.327–2.809). No publication bias was detected (Egger’s test, p=0.552).
Conclusion: This meta-analysis highlights TSR as a potential prognostic marker in HCC, where a high TSR is associated with improved survival. In GBC, the prognostic significance of TSR remains uncertain, possibly due to tumor heterogeneity and advanced-stage diagnoses. Given its prognostic value, TSR could be integrated into routine histopathological assessments, particularly in HCC, to enhance risk stratification and guide clinical decision-making.
Introduction
The tumor microenvironment (TME), consisting of non-cancerous cells and the surrounding extracellular matrix, plays a critical role in the development and spread of cancer. Within this complex environment, the tumor-stroma ratio (TSR) has emerged as a key factor in predicting outcomes in various solid tumors [1]. TSR refers to the proportion of tumor cells relative to the surrounding stromal tissue [2]. Studies have shown that a low TSR, indicating a high proportion of stromal tissue, is associated with worse clinical outcomes in several cancers, including colorectal, gastric, breast, oral tongue squamous cell carcinoma, and epithelial ovarian cancers [3-11]. In contrast, a higher TSR with less stromal content is generally linked to a better prognosis. The tumor stroma includes a mix of fibroblasts, endothelial cells, immune cells, and extracellular matrix, which interact with cancer cells and influence tumor growth and the potential for metastasis [1].
Hepatocellular carcinoma (HCC), one of the leading causes of cancer-related deaths globally, has a range of known prognostic factors such as tumor size, vascular invasion, and stage. Recently, TSR has been explored as an additional predictor of survival in HCC, though research results have been mixed, indicating a need for further study to clarify its role [12-13].
Gallbladder carcinoma (GBC) is a rare but aggressive cancer of the biliary tract. Often diagnosed at an advanced stage due to nonspecific symptoms and the absence of effective screening methods, GBC has a poor prognosis and limited treatment options. TSR, which reflects the balance between tumor cells and surrounding stromal tissue, has shown potential as a prognostic marker in GBC, with higher stromal content correlating with worse outcomes [14-15]. However, more research is needed to confirm the clinical value of TSR in GBC and to understand the mechanisms behind its impact.
This meta-analysis aims to evaluate the prognostic significance of TSR in both hepatocellular carcinoma and gallbladder carcinoma. By pooling data from existing studies, this analysis seeks to clarify the relationship between TSR and clinical outcomes in these cancers [13-17]. Through this review, we hope to provide a clearer understanding of TSR’s influence on prognosis in HCC and GBC, which could help improve clinical decision-making and guide future research on therapeutic strategies targeting the tumor stroma.
Materials and Methods
Protocol Registration
This protocol adheres to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) 2015 guidelines [18-19]. The review has been registered with PROSPERO (Registration Number: CRD42024547011).
Search Strategy
We searched PubMed, Scopus, and Web of Science databases without restrictions on language or publication date. The search terms included: “Hepatocellular carcinoma” OR “Liver cancer” OR “Gallbladder carcinoma” AND “Tumor stroma ratio.” Additionally, grey literature was explored via Google Scholar.
Eligibility Criteria
We included cohort studies (prospective and retrospective) and case-control studies that investigated the prognostic value of TSR in patients diagnosed with hepatocellular or gallbladder carcinoma.
• Participants: Patients diagnosed with hepatocellular or gallbladder carcinoma.
• Exposure: Patients categorized as having a high TSR (defined as TSR ≥50% based on the original study).
• Comparison: Comparisons were made between patients with high TSR and low TSR (with a cut-off of 50%).
• Outcome: The primary outcome of interest was overall survival in relation to TSR.
Study Selection
Three researchers (SA, SZ, and MF) independently screened and assessed the studies. Each citation was evaluated by at least two reviewers. Extracted data included the first author, country, publication year, number of patients, age, gender, and outcomes. Discrepancies were resolved through discussion until consensus was reached. The PRISMA flow diagram will be used to detail the article selection process.
Risk of Bias Assessment
Two researchers (SA and SZ) independently assessed the quality of the included studies using the Newcastle- Ottawa Scale [20].
Data Synthesis Strategy
A meta-analysis of hazard ratios (HR) will be conducted using STATA software (version 17, StataCorp). A random-effects model will be employed, and heterogeneity will be assessed using the I² statistic, Q test, and prediction interval. Subgroup analyses and meta-regression will explore sources of heterogeneity. Sensitivity analyses will also be performed to assess the robustness of the findings. For survival data, HRs and 95% confidence intervals (95% CI) will be extracted directly from the studies or estimated using established methods. Heterogeneity will be evaluated using Cochran’s Q test and Higgins I² statistic. When significant heterogeneity (p < 0.10) is identified, a random-effects model (DerSimonian-Laird method) will be applied; otherwise, a fixed-effects model (Mantel-Haenszel method) will be used. Meta-regression will explore potential sources of heterogeneity, including publication year, gender, cancer type, analysis methods, and TSR cut-off values. Sensitivity analyses will validate the robustness of the findings using the “metaninf” STATA command. Publication bias will be examined with Begg’s funnel plot and Egger’s linear regression test, with a p-value <0.05 considered statistically significant.
All statistical analyses will be performed using STATA software (version 17.0), with two-sided p-values.
Subgroup Analysis
Subgroup analyses will be conducted to assess the consistency of results across different patient subpopulations and to explore sources of heterogeneity [21]. Analyses will be stratified by variables such as analysis method and patient characteristics.
Results
A relevant search across Pubmed, Scopus and Web of Science identified 254 studies, of which 103 were duplicate records. In screening, 142 were excluded due to inapplicable information. Among the remaining nine articles, five were excluded for the following reasons- non-original article (n=3) and lack of supplementary data (n=2), thus leaving four studies for inclusion in the meta-analysis. The PRISMA diagram for the study selection is presented in Figure 1.
Figure 1. The PRISMA Diagram for the Study Selection.
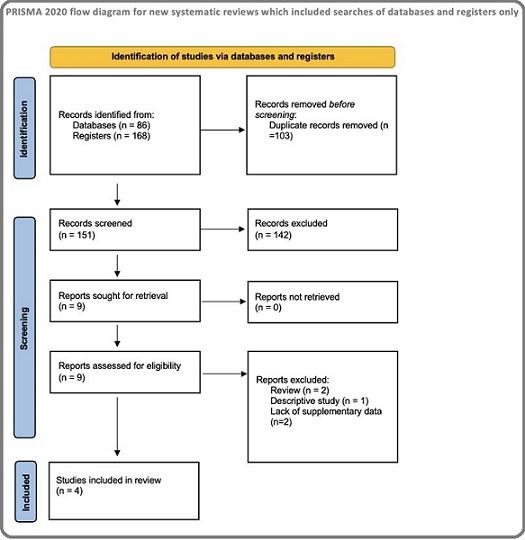
All of the included studies were retrospective cohorts, including patients diagnosed with hepatocellular or gall bladder cancer from India and China. The sample size for the studies varied between 51 and 300. In three of the four cohort studies, 50% was set as the cutoff for TSR, while in the remaining study, the optimal cutoff was calculated by a logrank test [Wang et al] (Table 1).
| Author and Publication year | Country | Organ | Criteria for High TSR (%) | Number of Patients | Tumor stroma ratio | |
| High | Low | |||||
| Goyal et al (2021) [16] | India | Gall bladder | 50 | 96 | 56 | 40 |
| Li et al (2017) [14] | China | Gall bladder | 50 | 51 | 32 | 19 |
| Lv et al (2015) [17] | China | Liver | 50 | 300 | 225 | 75 |
| Wang et al (2023) [13] | China | Liver | 52.5 | 95 | 69 | 26 |
The outcome of overall survival was reported in all the studies. Potential confounding factors, such as age, gender, tumor size, stage, grade, cirrhosis, and serum AFP, ALT, and AST, were adjusted in the multivariate analyses in the original studies. The Newcastle–Ottawa Scale score of the included studies was nine, indicating good study quality (Table 2).
| Representativeness of the exposed | Selection of the non-exposed | Ascertainment of exposure | Outcome not present at baseline | Comparability of cohorts | Assessment of outcome | Length of follow-up | Adequacy of follow-up | Total score | Quality | |
| Goyal et al -2021 [16] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High |
| Li et al (2017) [14] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High |
| Lv et al (2015) [17] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High |
| Wang et al -2023 [13] | 1 | 1 | 1 | 1 | 2 | 1 | 1 | 1 | 9 | High |
The combined analysis of all the studies indicated that low TSR significantly increased the risk of poorer outcomes (pooled HR = 1.963; 95% CI = 0.997–2.929; P= 0.480; random effects), with no observed heterogeneity (I² = 0.00%). In the subgroup analysis by cancer type, the effect was more pronounced in hepatocellular carcinoma, where low TSR was associated with worse outcomes (pooled HR = 2.566; 95% CI = 1.028–4.104; P = 0.697; random effects), with no heterogeneity (I² = 0.00%). In gallbladder cancer, the association was less clear, with low TSR showing a pooled HR of 1.818 (95% CI = -0.105–3.742; P = 0.245; random effects) and moderate heterogeneity (I² = 25.9%) (Table 3, Figure 2).
| Number of Subsets | Fixed Effect | Random Effect | I ² , % | Chi 2 | |
| (95% CI) | (95% CI) | ||||
| Overall | 4 | 1.963 (0.997, 2.929) | 1.963 (0.997, 2.929) | 0 | 0.48 |
| Hepatocellular | 2 | 2.566 (1.028, 4.104) | 2.566 (1.028, 4.104) | 0 | 0.697 |
| Gall bladder | 2 | 1.568 (0.327, 2.809) | 1.818 (-0.105, 3.742) | 25.9 | 0.245 |
Figure 2. Forest Plots of the Overall Outcome for Overall Survival (OS).
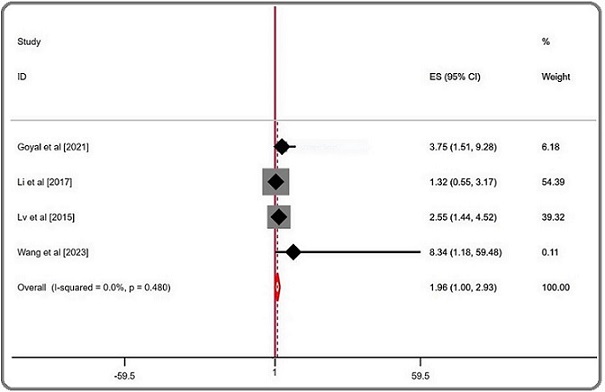
The between-study heterogeneity was low (I2: 0.00%), making it unnecessary to conduct meta-regression and sensitivity analysis.
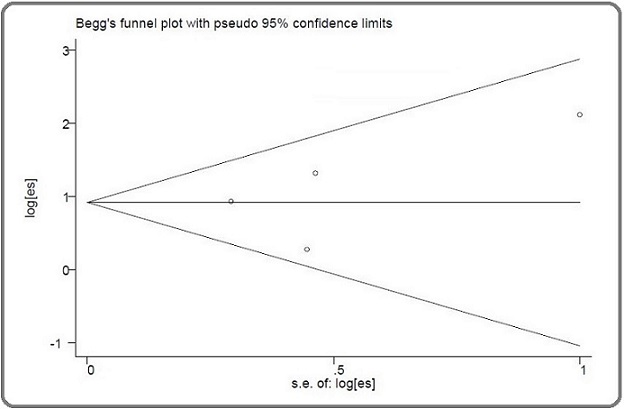
The Egger (p=0.552) and Begg (p=0.174) tests were not significant, indicating the absence of any publication bias (Figure 3). There was no heterogeneity in the present meta-analysis.
Figure 3. Begg’s funnel Plot for Publication Bias.
Discussion
This meta-analysis evaluated the prognostic significance of the TSR in hepatocellular carcinoma and gallbladder carcinoma, demonstrating that a high TSR (indicating a greater proportion of tumor cells relative to stromal content) is associated with improved overall survival. These findings reinforce TSR as a potential independent prognostic marker in solid tumors.
Our results align with the meta-analysis by Wu et al, which showed that stromal-rich tumors are linked to poorer clinical outcomes across multiple malignancies, including colorectal cancer (CRC), breast cancer (BC), esophageal carcinoma (EC), and non-small cell lung carcinoma (NSCLC). In their study, low TSR was significantly associated with worse overall survival in CRC (HR = 2.25, 95% CI = 1.40–3.61), NSCLC (HR = 1.77, 95% CI = 1.33–2.35), HCC (HR = 2.25, 95% CI = 1.47–3.43), BC (HR = 1.52, 95% CI =1.23–1.88), and EC (HR = 2.56, 95% CI = 1.72–3.79) [22]. Our findings similarly indicate that a higher stromal proportion (low TSR) correlates with a worse prognosis in HCC (HR = 2.566, 95% CI = 1.028–4.104), supporting the concept that tumor-stromal interactions significantly influence survival. However, the effect in GBC was less pronounced (HR = 1.568, 95% CI = 0.327–2.809), contrasting with the strong association observed in other malignancies.
The meta-analysis by Pyo et al further supports the prognostic significance of TSR across various cancers. Their results revealed that high TSR was associated with improved survival in most cancers, including colorectal (HR = 0.588, 95% CI = 0.429–0.804), breast (HR = 0.630, 95% CI = 0.443–0.896), esophageal (HR = 0.406, 95% CI = 0.294–0.559), and stomach (HR = 0.456, 95% CI = 0.324–0.641) cancers. However, in endometrial (HR = 2.510, 95% CI = 1.223–5.152) and pancreatic cancers (HR = 1.957, 95% CI = 1.443–2.654), high TSR was correlated with a significantly worse prognosis. Notably, gallbladder carcinoma (HR = 0.568, 95% CI = 0.276–1.169) and liver cancer (HR = 0.538, 95% CI = 0.262–1.105) showed weaker associations, aligning with our findings that the prognostic impact of TSR in these cancers is less pronounced [23].
The stronger association in HCC could be attributed to the established role of fibrosis and cirrhosis in hepatocarcinogenesis. Chronic liver disease, a common precursor to HCC, leads to extensive stromal remodeling, with activated hepatic stellate cells and myofibroblasts contributing to tumor progression. Given this background, TSR may be particularly useful in HCC as it reflects the balance between tumor expansion and stromal resistance. Conversely, the weaker association in GBC may stem from the heterogeneity of gallbladder tumors, which encompass multiple histological subtypes with varying degrees of desmoplasia, immune cell infiltration, and extracellular matrix composition. Additionally, since GBC is frequently diagnosed at an advanced stage, stromal interactions may already be well-established, potentially reducing TSR’s prognostic utility [24].
The biological mechanisms underlying this phenomenon lie in the composition and function of the tumor stroma. Tumor-associated fibroblasts, immune cells, endothelial cells, and extracellular matrix components interact with cancer cells to promote tumor progression, immune evasion, and therapy resistance. A dense stromal environment may contribute to extracellular matrix deposition and fibrosis, creating a physical and biochemical barrier that limits systemic therapy effectiveness, facilitates tumor invasion, and promotes a pro-inflammatory microenvironment that enhances malignancy [25].
These findings underscore the importance of incorporating TSR into routine histopathological evaluation. Given its prognostic value, TSR could be integrated into existing tumor staging systems to refine risk stratification, particularly in HCC. Identifying patients with high stromal content who are at greater risk for poor outcomes may help tailor treatment approaches, including intensified surveillance, adjuvant therapies, and strategies targeting stromal components. From a therapeutic perspective, these results highlight the potential for targeting the tumor stroma in HCC and GBC treatment. Stromal-modulating agents, such as antifibrotic drugs, immune checkpoint inhibitors, and agents disrupting tumor-stroma crosstalk, may improve patient outcomes. Future clinical trials should explore whether TSR can serve as a biomarker for selecting patients who would benefit from stromal-targeting therapies.
Despite these insights, this meta-analysis has several limitations. First, all included studies were retrospective, introducing selection bias and limiting generalizability. Prospective validation in larger, multi-center cohorts is necessary to establish TSR as a robust prognostic marker. Second, heterogeneity in TSR assessment methods across studies presents a challenge. While three of the four studies used a 50% cutoff, one applied an optimized threshold derived from a log-rank test, potentially affecting comparability. Standardized criteria for TSR measurement, including automated digital pathology approaches, should be established to enhance reproducibility. Additionally, although confounding factors such as tumor size, stage, and serum biomarkers were adjusted for in multivariate analyses, other potential prognostic variables (e.g., molecular subtypes, immune infiltration, and treatment regimens) were not uniformly accounted for across studies. Future research should integrate these factors to further refine TSR’s prognostic utility. Lastly, while publication bias was not statistically significant in Egger’s and Begg’s tests, the limited number of studies constrains the power of these assessments. Larger-scale studies with diverse populations are needed to confirm these findings and assess whether TSR can be applied consistently across different ethnic and geographic cohorts.
In conclusion, this meta-analysis highlights TSR as a promising prognostic indicator in both HCC and GBC, with a stronger predictive value observed in HCC. Given its potential clinical relevance, incorporating TSR into routine histopathological assessments may improve prognostic accuracy and inform therapeutic decision-making. However, further prospective studies are needed to validate its utility, standardize its measurement, and explore its role in guiding stromal-targeting treatment strategies. The integration of TSR into cancer prognostication models could enhance precision medicine efforts, ultimately improving patient outcomes in HCC and GBC.
Acknowledgments
This systematic review is part of an internship program supported by the “Health Economics and Finance Research Group University of Sharjah, Sharjah, United Arab Emirates” and implemented by the West Asian Organization for Cancer Prevention (APOCP’s West Asia Chapter).
Competing interests
The authors have no conflicts of interest to declare.
Human Ethics and Consent to Participate
Not applicable
Data availability statement
Supplementary data are available on request from the corresponding author.
Author contribution statement
SA, MF, SZ, AMJ jointly contributed to the study aims, research design and methodology. SA and SZ produced the first draft of the article outline with the guidance of MF. SA and MF designed the search strategy. All authors contributed substantially to the manuscript and critically revised the content. All authors read and approved the final version of the manuscript.
References
- The tumor microenvironment Anderson NM , Simon MC . Current biology: CB.2020;30(16). CrossRef
- Standardized Assessment of the Tumor-Stroma Ratio in Colorectal Cancer: Interobserver Validation and Reproducibility of a Potential Prognostic Factor Souza da Silva RM , Queiroga EM , Paz AR , Neves FFP , Cunha KS , Dias EP . Clinical Pathology (Thousand Oaks, Ventura County, Calif.).2021;14. CrossRef
- Tumor Stroma Ratio and Its Significance in Locally Advanced Colorectal Cancer Sullivan L, Pacheco RR , Kmeid M, Chen A, Lee H. Current Oncology (Toronto, Ont.).2022;29(5). CrossRef
- The value of the tumour-stroma ratio for predicting neoadjuvant chemoradiotherapy response in locally advanced rectal cancer: a case control study Liang Y, Zhu Y, Lin H, Zhang S, Li S, Huang Y, Liu C, et al . BMC cancer.2021;21(1). CrossRef
- Tumour-stroma ratio and 5-year mortality in gastric adenocarcinoma: a systematic review and meta-analysis Kemi N, Eskuri M, Kauppila JH . Scientific Reports.2019;9(1). CrossRef
- Tumour stroma ratio is a potential predictor for 5-year disease-free survival in breast cancer Yan D, Ju X, Luo B, Guan F, He H, Yan H, Yuan J. BMC cancer.2022;22(1). CrossRef
- Prognostic Significance of the Tumor-Stromal Ratio in Invasive Breast Cancer and a Proposal of a New Ts-TNM Staging System Xu Q, Yuan J, Chen Y, Zhang H, Wang L, Xiong B. Journal of Oncology.2020;2020. CrossRef
- Prognostic value of tumor-stroma ratio in oral carcinoma: Role of cancer-associated fibroblasts Qiu J, Jiang E, Shang Z. Oral Diseases.2023;29(5). CrossRef
- Prognostic Significance of the Tumor-Stroma Ratio in Epithelial Ovarian Cancer Chen Y, Zhang L, Liu W, Liu X. BioMed Research International.2015;2015. CrossRef
- Prognostic Efficacy of Tumor-Stroma Ratio in Women With Breast Cancer: A Meta-Analysis of Cohort Studies Jiang P, Chen Y, Liu B. Frontiers in Oncology.2021;11. CrossRef
- Prognostic and clinicopathological significance of tumor-stroma ratio in head and neck squamous cell carcinoma: A systematic review Morais E.-F., Morais H.-G., Martins H.-D., Carlan L.-M., Costa A.-D., Freitas R.-D.. Medicina Oral, Patologia Oral Y Cirugia Bucal.2022;27(4). CrossRef
- Tumour budding and tumour-stroma ratio in hepatocellular carcinoma Kairaluoma V, Kemi N, Pohjanen V, Saarnio J, Helminen O. British Journal of Cancer.2020;123(1). CrossRef
- Tumor-stroma ratio predicts prognosis and PD-L1 expression in hepatocellular carcinoma Wang D, Luo J, Tao Y. BMC cancer.2023;23(1). CrossRef
- Prognostic significance of the tumor-stroma ratio in gallbladder cancer Li H., Yuan S. L., Han Z. Z., Huang J., Cui L., Jiang C. Q., Zhang Y.. Neoplasma.2017;64(4). CrossRef
- Is the tumor-stroma ratio a prognostic factor in gallbladder cancer? Uzun MA , Tilki M, Gönültaş A, Aker F, Kayaoglu SA , Okuyan GC . Revista Da Associacao Medica Brasileira (1992).2022;68(5). CrossRef
- Prognostic significance of tumour budding, tumour-stroma ratio and desmoplastic stromal reaction in gall bladder carcinoma Goyal S, Banga P, Meena N, Chauhan G, Sakhuja P, Agarwal AK . Journal of Clinical Pathology.2023;76(5). CrossRef
- Tumor-stroma ratio is a prognostic factor for survival in hepatocellular carcinoma patients after liver resection or transplantation Lv Z, Cai X, Weng X, Xiao H, Du C, Cheng J, Zhou L, et al . Surgery.2015;158(1). CrossRef
- Prognostic Significance of Tumor-Stroma Ratio in Hepatocellular and Gall Bladder Carcinoma: Protocol for a Systematic Review and Meta-analysis Ahuja S, Zaheer S, Fattahi-Darghlou M, Jarrahi AM , Nazari SSH , Marzouqi AMA , Rahman SA , et al . Asian Pacific Journal of Environment and Cancer.2025. CrossRef
- Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA . Systematic Reviews.2015;4(1). CrossRef
- The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analysis, 2017. Available: www. ohri. ca/ programs/ clinical_ epidemiology/oxford. asp Wells BS GA , O'Connell D, Peterson J, Welch V, Losos M, Tugwell P. .
- Quantifying heterogeneity in a meta-analysis Higgins JPT , Thompson SG . Statistics in Medicine.2002;21(11). CrossRef
- Association between tumor-stroma ratio and prognosis in solid tumor patients: a systematic review and meta-analysis Wu J, Liang C, Chen M, Su W. Oncotarget.2016;7(42). CrossRef
- Significance of Tumor-Stroma Ratio (TSR) in Predicting Outcomes of Malignant Tumors Pyo J, Kim NY , Min K, Kang D. Medicina (Kaunas, Lithuania).2023;59(7). CrossRef
- Origin and role of hepatic myofibroblasts in hepatocellular carcinoma Yavuz BG , Pestana RC , Abugabal YI , Krishnan S, Chen J, Hassan MM , Wolff RA , et al . Oncotarget.2020;11(13). CrossRef
- Cancer-Associated Fibroblasts: The Origin, Biological Characteristics and Role in Cancer-A Glance on Colorectal Cancer Fotsitzoudis C, Koulouridi A, Messaritakis I, Konstantinidis T, Gouvas N, Tsiaoussis J, Souglakos J. Cancers.2022;14(18). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2025
Author Details