From ROS to Tumorigenesis: Understanding the Oxidative Pathways in Cervical Cancer Progression
Download
Abstract
Cervical cancer remains a leading cause of cancer-related morbidity and mortality among women worldwide. Although persistent infection with high-risk human papillomaviruses (HPV) constitutes the principal etiological agent, accumulating evidence implicates oxidative stress as a pivotal co-factor in the initiation, promotion, and progression of cervical carcinogenesis. Reactive oxygen species (ROS), generated endogenously and exogenously, inflict cumulative genetic and epigenetic alterations, foster a pro-inflammatory tumor microenvironment, and promote malignant transformation. While endogenous antioxidant defense mechanisms initially mitigate ROS-mediated damage, their failure or exhaustion during disease evolution facilitates tumor progression and therapeutic resistance. This review critically examines the intricate interplay between oxidative stress and antioxidant responses throughout the stages of cervical cancer development. Furthermore, it explores emerging redox-targeted therapeutic strategies, emphasizing the dualistic role of antioxidants in cervical neoplasia and the challenges of modulating oxidative balance in clinical settings. A deeper understanding of redox dynamics may inform novel preventive and therapeutic interventions against cervical cancer.
1. Introduction
Given the multifactorial nature of cervical carcinogenesis, a comprehensive understanding of oxidative stress pathways and antioxidant defense mechanisms is crucial to unraveling the complex biological events that underlie disease initiation, promotion, and progression [1]. Although HPV vaccination and screening programs have significantly reduced cervical cancer incidence in high-resource settings, therapeutic outcomes for advanced or recurrent disease remain suboptimal, with five-year survival rates dropping dramatically once the disease becomes invasive [2]. Emerging studies suggest that oxidative stress not only contributes to the malignant transformation of HPV-infected cervical epithelial cells but also influences tumor aggressiveness, therapeutic resistance, and immune evasion [3].
The dynamic interplay between reactive oxygen species (ROS) generation and antioxidant responses shapes the tumor microenvironment and determines the trajectory of neoplastic progression [4]. Dysregulated redox homeostasis leads to sustained DNA damage, chronic inflammation, angiogenesis, and epithelial mesenchymal transition (EMT), thereby exacerbating tumor development and metastasis [5]. Conversely, the manipulation of oxidative stress pathways presents a promising therapeutic strategy, wherein both antioxidant supplementation and pro-oxidant therapies aimed at inducing lethal oxidative damage in cancer cells are under active investigation [6].
This review aims to critically examine the role of oxidative stress and antioxidant defense mechanisms across the different stages of cervical cancer development, from precancerous lesions to invasive and metastatic disease. By synthesizing current evidence, it seeks to illuminate the molecular pathways through which redox imbalance promotes cervical tumorigenesis and to explore the translational potential of targeting redox homeostasis in preventive and therapeutic interventions. Understanding these mechanisms may facilitate the identification of novel biomarkers, inform personalized therapeutic approaches, and ultimately contribute to improving clinical outcomes for patients with cervical cancer.
1.1 Oxidative Stress and Reactive Oxygen Species (ROS): General Concepts
Reactive oxygen species (ROS) are chemically reactive molecules derived from molecular oxygen, encompassing a range of free radicals such as superoxide anion (O₂•⁻), hydroxyl radical (•OH), and non-radical derivatives like hydrogen peroxide (H₂O₂) and singlet oxygen (¹O₂) [7].
These species are generated as natural by-products of cellular metabolism, particularly through mitochondrial oxidative phosphorylation, and play essential roles in physiological signaling pathways regulating cell proliferation, differentiation, and immune responses [8].
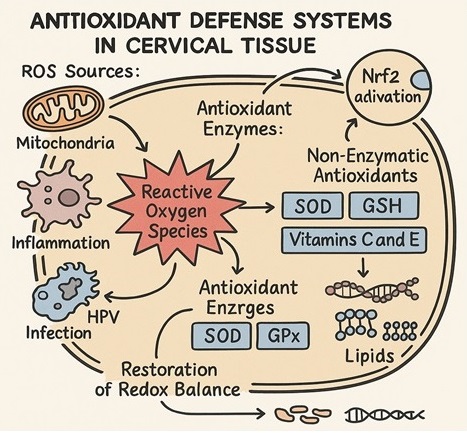
Under homeostatic conditions, intracellular ROS levels are tightly controlled by a network of antioxidant defense systems, including enzymatic antioxidants such as superoxide dismutase (SOD), catalase (CAT), and glutathione peroxidase (GPx), as well as non-enzymatic antioxidants like glutathione (GSH), vitamins C and E, and thioredoxin [9]. A delicate balance between ROS production and antioxidant activity is critical for maintaining cellular integrity and function.
Oxidative stress arises when there is an imbalance favoring ROS generation over antioxidant defenses, resulting in excessive accumulation of reactive species that can damage cellular macromolecules [10]. DNA oxidation, lipid peroxidation, and protein modifications induced by ROS contribute to genomic instability, impaired cellular signaling, and activation of pro-inflammatory cascades, all of which are implicated in carcinogenesis [11].
Although ROS were historically viewed primarily as harmful by-products of metabolism, recent research has underscored their dual role as both signaling molecules and drivers of pathological processes. At moderate levels, ROS participate in redox signaling critical for adaptation and survival, whereas chronic or overwhelming ROS exposure can trigger cellular senescence, apoptosis, or malignant transformation, depending on the cellular context [12]. In cancer development, sustained oxidative stress not only induces DNA damage and mutagenesis but also promotes tumor cell proliferation, survival, angiogenesis, and metastasis [13].
Thus, understanding the generation, regulation, and consequences of oxidative stress is fundamental to elucidating its role in cervical carcinogenesis, where persistent HPV infection and inflammatory responses create a microenvironment conducive to ROS accumulation and redox imbalance [14].
2. ROS in Cervical Carcinogenesis
2.1 Initiation Stage: HPV Infection, ROS Production, and Genomic Instability ROS
Persistent infection with high-risk human papillomaviruses (HPV), particularly HPV-16 and HPV-18, initiates the oncogenic process in cervical epithelial cells. The E6 and E7 viral oncoproteins not only inactivate pivotal tumor suppressors such as p53 and pRb, but also induce profound metabolic reprogramming and mitochondrial dysfunction, leading to excessive generation of intracellular ROS [15].
Mechanistically, HPV E6 downregulates p53-mediated antioxidant responses, diminishing the expression of genes involved in ROS detoxification, such as catalase and glutathione peroxidase [3]. Concurrently, E7 interacts with mitochondrial proteins to enhance mitochondrial ROS leakage [4]. The net result is elevated oxidative stress, which inflicts DNA lesions including 8-oxo-2’- deoxyguanosine (8-oxo-dG), single-strand breaks, and double-strand breaks [16].
Persistent oxidative DNA damage promotes genomic instability a hallmark of cancer facilitating chromosomal aberrations, aneuploidy, and integration of HPV DNA into host genomic loci. Integration events further dysregulate oncogene expression and potentiate malignant transformation [17]. Additionally, ROS-mediated oxidative damage alters telomere maintenance, accelerates telomere attrition, and fosters cellular immortalization, a critical step in cervical carcinogenesis [18].
2.2 Promotion Stage: Chronic Inflammation, Oxidative Stress, and Dysplastic Progression
The promotional phase of cervical carcinogenesis is characterized by chronic inflammation and sustained oxidative stress [19]. Persistent HPV infection triggers an inflammatory response dominated by pro-inflammatory cytokines (e.g., TNF-α, IL-1β, IL-6) and immune cell infiltration, particularly by neutrophils and macrophages. These immune cells are major sources of ROS and reactive nitrogen species (RNS), amplifying the oxidative burden in cervical tissue [20].
ROS actively modulate key oncogenic signaling pathways during this stage:
• Activation of NF-κB signaling promotes the transcription of genes involved in proliferation, survival, angiogenesis, and immune evasion [21].
• STAT3 activation, often stimulated by IL-6, fosters tumor-promoting inflammation and cellular proliferation [22].
• ROS also inhibit the activity of phosphatases (e.g., PTEN), leading to hyperactivation of PI3K/Akt/mTOR signaling, which promotes cell survival, metabolic adaptation, and resistance to apoptosis [23].
Oxidative stress-induced epigenetic modifications further contribute to neoplastic progression. ROS influence DNA methyltransferases (DNMTs) and histone-modifying enzymes, resulting in the silencing of tumor suppressor genes and activation of oncogenic pathways [24]. Moreover, oxidative stress promotes epithelial–mesenchymal transition (EMT) through upregulation of transcription factors such as Snail, Slug, and Twist, enhancing migratory and invasive capacities of cervical epithelial cells. This EMT process is tightly linked to the acquisition of cancer stem cell-like properties, contributing to tumor heterogeneity and therapeutic resistance [25].
2.3 Progression Stage: Malignant Transformation, Angiogenesis, and Metastasis
In the malignant progression stage, oxidative stress continues to orchestrate key events that promote tumor aggressiveness. ROS stabilize hypoxia-inducible factor-1 alpha (HIF-1α) even under normoxic conditions, a phenomenon termed “pseudohypoxia,” leading to the overexpression of angiogenic factors such as vascular endothelial growth factor (VEGF) [26].
ROS-mediated activation of the matrix metalloproteinase (MMP) family, particularly MMP- 2 and MMP-9, facilitates extracellular matrix (ECM) degradation, enabling tumor invasion and metastasis [27]. Furthermore, chronic oxidative stress impairs the cytotoxic function of tumor-infiltrating lymphocytes (TILs) and natural killer (NK) cells, thereby promoting immune evasion [28].
Importantly, elevated ROS levels confer resistance to chemotherapeutic agents and radiotherapy by enhancing DNA repair capacity and activating anti-apoptotic pathways such as Bcl-2 family proteins and NF-κB-mediated survival signaling; consequently, oxidative stress not only accelerates tumor progression but also poses a formidable barrier to effective cancer treatment [29].
Schematic Summary
In summary, ROS act as critical regulators across all stages of cervical carcinogenesis by:
• Inducing DNA damage and genomic instability (initiation),
• Promoting inflammatory signaling, survival pathways, and EMT (promotion),
• Enhancing angiogenesis, immune evasion, metastasis, and therapy resistance (progression).
Understanding these oxidative mechanisms is paramount for identifying novel therapeutic targets and improving patient outcomes (Figure 1).
Figure 1. Role of ROS in HPV-Induced Cervical Carcinogenesis Pathway.
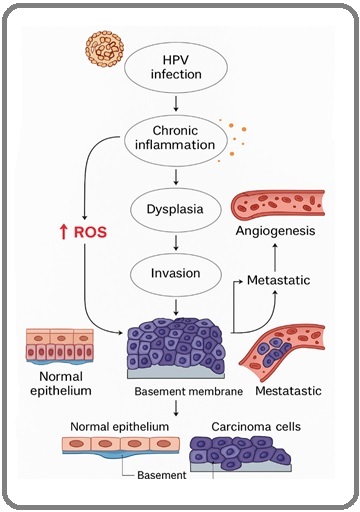
3. Antioxidant Systems in Cervical Tissue
3.1 Overview of Cellular Antioxidant Defense Mechanisms
The cervical epithelium, like other rapidly renewing tissues, is continuously exposed to endogenous and exogenous sources of oxidative stress, to counteract the deleterious effects of reactive oxygen species (ROS), a sophisticated network of antioxidant defenses has evolved, encompassing both enzymatic and non-enzymatic components [30]. The enzymatic antioxidants, such as superoxide dismutases (SODs), catalase (CAT), and glutathione peroxidases (GPx), form the first line of defense against ROS accumulation [31]. Superoxide dismutases catalyze the dismutation of superoxide anions into hydrogen peroxide, while catalase subsequently decomposes hydrogen peroxide into water and molecular oxygen, preventing the formation of hydroxyl radicals [32]. Glutathione peroxidases reduce hydrogen peroxide and lipid hydroperoxides by utilizing glutathione (GSH) as a substrate [33].
Complementing the enzymatic systems are non-enzymatic antioxidants, including glutathione, vitamins C and E, thioredoxin, and peroxiredoxins. These molecules act as scavengers, intercepting ROS before they can damage critical cellular structures. Together, these antioxidant systems maintain tightly regulated redox homeostasis [30]. The dynamic interplay between antioxidant enzymes and redox-sensitive transcription factors, notably Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2), ensures a rapid and robust cellular response to oxidative fluctuations [34] (Figure 2).
Figure 2. Antioxidant Defense Mechanisms Against ROS in Cervical Tissue.
3.2 Antioxidant Responses in Early-Stage Cervical Lesions
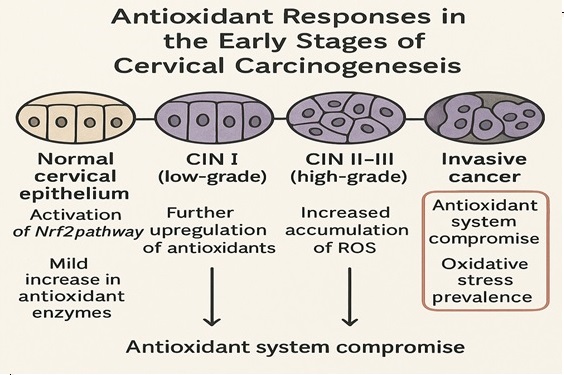
During the initial stages of cervical carcinogenesis, particularly in low-grade squamous intraepithelial lesions (LSIL), an upregulation of antioxidant defenses is often observed [35]. Persistent high-risk HPV infection induces ROS production, and in response, cells increase the expression and activity of antioxidant enzymes such as SOD, catalase, and GPx [19].
Activation of the Nrf2 signaling pathway is central in this adaptive response. Under oxidative conditions, Nrf2 translocates to the nucleus and induces transcription of antioxidant response element (ARE)-driven genes, including HO-1, NQO1, and GCLC(36). This antioxidant upregulation temporarily mitigates oxidative damage but may paradoxically facilitate the survival of HPV-infected cells harboring oncogenic mutations, aiding in their clonal expansion [37].
3.3 Collapse of Antioxidant Defenses During Cancer Progression
As lesions advance toward high-grade squamous intraepithelial lesions (HSIL) and invasive cervical carcinoma, antioxidant defenses collapse [38]. Chronic oxidative stress overwhelms enzymatic systems, leading to reduced activity of SOD, CAT, and GPx. Moreover, epigenetic silencing of antioxidant genes such as SOD2 and GPX3 via promoter hypermethylation has been reported in cervical cancer tissues [39].
The role of Nrf2 becomes increasingly complex; sustained activation can promote tumor growth and therapeutic resistance by upregulating survival pathways. Additionally, mitochondrial dysfunction characterized by depletion of Mn-SOD and GSH exacerbates oxidative damage, fostering a malignant phenotype [40]. The progressive loss of redox control facilitates tumor aggression, metastasis, and therapy resistance (Figure 3) [5].
Figure 3. Progressive Antioxidant System Dysregulation During Cervical Carcinogenesis.
4. Therapeutic Implications: Targeting Oxidative Stress
4.1 Antioxidants as Preventive and Therapeutic Agents
Given the pivotal role of oxidative stress in cervical carcinogenesis, considerable attention has been directed toward the use of antioxidants as preventive and therapeutic agents [41]. Antioxidants aim to restore redox balance, reduce ROS-mediated DNA damage, and suppress inflammation, thereby potentially halting the progression of precancerous lesions or sensitizing tumors to conventional therapies [42].
Several epidemiological studies have suggested that dietary intake of antioxidant-rich foods, particularly those containing vitamins C and E, β-carotene, and polyphenols, is associated with a reduced risk of cervical intraepithelial neoplasia and cervical cancer [43]. Vitamin C, a potent water-soluble antioxidant, scavenges a wide range of ROS, regenerates vitamin E, and supports immune function, while vitamin E primarily protects cellular membranes from lipid peroxidation [37]. Natural polyphenolic compounds, such as curcumin, resveratrol, and epigallocatechin gallate (EGCG), exhibit strong antioxidant and anti-inflammatory properties and have demonstrated inhibitory effects on HPV oncogene expression and cervical cancer cell proliferation in preclinical studies [44].
In addition to dietary sources, pharmacological supplementation with antioxidants has been explored as a strategy for cervical cancer prevention. Randomized clinical trials evaluating micronutrient supplementation in women with cervical dysplasia have yielded mixed results, reflecting variability in study designs, antioxidant formulations, and patient populations [45]. Nonetheless, the concept of antioxidant-based chemoprevention remains attractive, particularly in high-risk populations with persistent HPV infection and evidence of oxidative DNA damage.
4.2 Pro-oxidant Therapies: Inducing ROS Overload in Cancer Cells
Paradoxically, while antioxidants aim to neutralize ROS, another therapeutic approach exploits the vulnerability of cancer cells to oxidative stress by inducing ROS overload [46]. Cancer cells, including cervical cancer cells, often operate near the threshold of tolerable oxidative stress due to their high metabolic rates and oncogene-driven ROS production. By further elevating ROS levels beyond this threshold, pro-oxidant therapies can selectively induce cancer cell apoptosis while sparing normal cells with intact antioxidant defenses [47].
Several chemotherapeutic agents, such as cisplatin and paclitaxel, exert their cytotoxic effects at least in part by generating ROS and triggering oxidative stress-mediated cell death pathways [48]. Additionally, novel compounds designed to disrupt redox homeostasis, including inhibitors of thioredoxin reductase and glutathione synthesis, are being investigated for their potential to synergize with conventional therapies [47].
Photodynamic therapy (PDT) represents another pro-oxidant strategy with growing interest in cervical cancer management. PDT involves the administration of a photosensitizing agent followed by light activation, resulting in localized ROS generation and targeted tumor destruction [49]. Clinical studies have demonstrated the efficacy of PDT in treating cervical intraepithelial neoplasia, with the added advantage of preserving cervical tissue integrity compared to surgical excision [50].
4.3 Challenges and Controversies in Redox-Targeted Therapies
Despite the promising rationale, targeting oxidative stress in cervical cancer therapy presents several challenges and unresolved controversies. One major concern is the dual role of antioxidants and ROS in cancer biology [51]. While antioxidants may protect normal cells and prevent malignant transformation, excessive antioxidant supplementation could inadvertently shield cancer cells from oxidative damage induced by chemotherapy or radiation therapy, potentially reducing treatment efficacy. The timing, dosage, and molecular context of antioxidant administration are critical factors influencing therapeutic outcomes [52]. Interventions during the early stages of cervical carcinogenesis may offer protective benefits, whereas antioxidant use in established tumors may require careful modulation to avoid promoting tumor survival. Furthermore, the heterogeneity of redox states among different tumor subtypes and individual patients complicates the design of universal antioxidant strategies [53]. Similarly, pro-oxidant therapies must balance efficacy with safety, as excessive ROS production can cause collateral damage to normal tissues and exacerbate inflammation, potentially contributing to long-term toxicity and secondary malignancies [54]. Identifying biomarkers of oxidative stress and redox vulnerability is essential for patient stratification and personalized therapeutic approaches [55].
4.4 Future Perspectives: Redox Modulation as a Therapeutic Strategy
Future research efforts should focus on elucidating the molecular determinants of redox balance in cervical cancer and developing targeted interventions that selectively modulate oxidative stress pathways. Combining redox-targeting agents with conventional therapies, such as chemotherapy, radiotherapy, and immunotherapy, holds particular promise in overcoming therapeutic resistance and improving clinical outcomes [56]. Advances in nanotechnology offer innovative solutions for redox modulation, such as nanoparticle-based delivery systems that selectively deliver antioxidants or pro-oxidant agents to tumor tissues, minimizing systemic toxicity [57, 58].
In conclusion, while redox-targeted therapies hold significant promise, a nuanced understanding of the complex interplay between oxidative stress, antioxidant defenses, and tumor biology is essential for translating these strategies into effective clinical interventions for cervical cancer (Table 1).
| Therapy/Agent | Mechanism of Action | Stage Targeted | Benefits | Limitations |
| Vitamin C | Scavenges ROS, supports DNA repair, and regenerates vitamin E | Precancerous lesions (CIN I/II) | Reduces oxidative DNA damage; boosts immune response | Poor stability; low tissue penetration |
| Vitamin E | Lipid-soluble antioxidant; prevents lipid peroxidation | Early stages | Protects membranes; synergizes with vitamin C | Risk of high-dose toxicity |
| Curcumin | Antioxidant, anti-inflammatory, suppresses HPV oncogenes | Precancerous and early invasive stages | Multifunctional; low systemic toxicity | Poor bioavailability; needs carriers |
| Resveratrol | Scavenges ROS, modulates p53 and Nrf2 | Early to moderate stages | Inhibits proliferation and promotes apoptosis | Rapid metabolism requires high doses |
| Cisplatin | Induces ROS accumulation, causes DNA crosslinking | Advanced invasive carcinoma | Highly effective cytotoxic agent | Chemoresistance development; nephrotoxicity |
| Paclitaxel | Promotes ROS generation; inhibits mitosis | Advanced carcinoma and metastasis | Effective in combination with chemotherapy | Peripheral neuropathy risk |
| Photodynamic Therapy (PDT) | Local ROS burst upon photosensitizer activation | High-grade CIN and early carcinoma | Tissue-sparing, selective tumor targeting | Requires light access; limited depth |
| Pro-oxidant nanoparticles (e.g., ZnO, Fe₃O₄) | Induce ROS-mediated apoptosis selectively in tumor cells | Experimental/preclinical stages | High selectivity; potential for minimal side effects | Need further validation; toxicity concerns |
5. Future Directions
The growing recognition of oxidative stress as a central player in cervical carcinogenesis has opened new avenues for research and therapeutic innovation. A deeper understanding of the molecular crosstalk between reactive oxygen species (ROS), antioxidant defenses, and oncogenic pathways will be critical for identifying novel biomarkers and developing more effective intervention strategies [4].
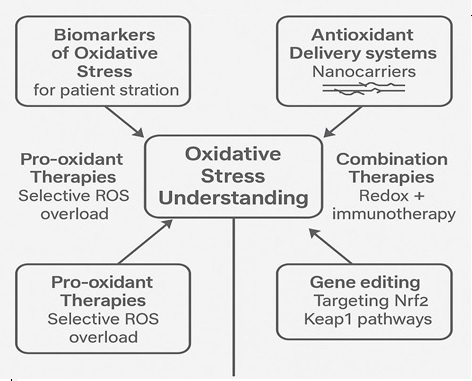
One promising direction involves the identification and validation of oxidative stress biomarkers that can predict disease progression, treatment response, and prognosis. Quantifying oxidative DNA damage products, lipid peroxidation markers, or antioxidant enzyme activities in cervical tissues or circulating fluids could enable risk stratification of patients and inform personalized therapeutic decisions [59]. Integrating redox biomarkers into screening programs may also enhance the early detection of precancerous lesions and allow timely preventive interventions [25].
Therapeutically, the development of selective redox modulators offers a compelling approach to complement existing treatments. Agents capable of fine-tuning oxidative stress levels either by augmenting antioxidant defenses in normal cells or by inducing lethal oxidative injury in tumor cells are being actively investigated [60]. Combining redox-targeting strategies with conventional therapies, such as chemotherapy, radiotherapy, and immunotherapy, holds particular promise in overcoming therapeutic resistance and improving clinical outcomes [56].
Advances in nanotechnology offer innovative solutions for redox modulation, such as nanoparticle-based delivery systems that selectively deliver antioxidants or pro-oxidant agents to tumor tissues, minimizing systemic toxicity and enhancing therapeutic efficacy [57]. Moreover, the integration of redox biomarkers into clinical practice could facilitate the monitoring of therapeutic responses and early identification of treatment resistance [58].
Furthermore, elucidating the interplay between oxidative stress and immune responses in the cervical tumor microenvironment represents an exciting frontier. Understanding how ROS modulates immune cell function could reveal new strategies to enhance antitumor immunity and synergize with immune checkpoint inhibitors [61].
Ultimately, advancing our knowledge of redox biology in cervical cancer will require interdisciplinary collaborations integrating molecular biology, oncology, immunology, and bioengineering. Such efforts are essential to translate conceptual insights into tangible benefits for patients, moving toward more precise, effective, and personalized therapies in the fight against cervical cancer (Figure 4).
Figure 4. Future Directions in Oxidative Stress-Targeted Therapies.
In conclusion, cervical cancer remains a major global health burden, with oxidative stress recognized as a crucial co-factor alongside persistent HPV infection. Reactive oxygen species (ROS) contribute to genomic instability and disease progression, while the collapse of antioxidant defenses accelerates malignancy and therapeutic resistance. Redox-targeted therapies, including antioxidants and pro-oxidants, offer promise but require careful, context-specific application. Future research should focus on identifying oxidative biomarkers and developing integrated redox-based strategies. Understanding oxidative dynamics may ultimately lead to more effective, personalized interventions in cervical
cancer care.
Clinical trial
not applicable.
Clinical trial number
not applicable.
Acknowledgments
Statement of Transparency and Principals:
• Author declares no conflict of interest
• Study was approved by Research Ethic Committee of author affiliated Institute.
• Study’s data is available upon a reasonable request.
• All authors have contributed to implementation of this research.
References
- Evaluation of Oxidative Stress, Anti-Oxidant, Vitamins and Co-Factor Elements in The Sera of Gastric Cancer in Iraqi Patients Mahdi M, Dawood Y, Sabah R, Abd Al-Rahman S. Asian Pacific Journal of Cancer Prevention.2024;25(10). CrossRef
- Evaluating the implementation of cervical cancer screening programs in low-resource settings globally: a systematized review Dykens JA , Smith JS , Demment M, Marshall E, Schuh T, Peters K, Irwin T, et al . Cancer Causes & Control.2020;31(5). CrossRef
- Oxidative Stress and DNA damage as Promoting Factors of HPV Integration Katerji M. Loma Linda University Electronic Theses, Dissertations & Projects.2021.
- Role of Reactive Oxygen Species in Cancer Progression: Molecular Mechanisms and Recent Advancements Aggarwal V, Tuli H, Varol A, Thakral F, Yerer M, Sak K, Varol M, et al . Biomolecules.2019;9(11). CrossRef
- Redox Dysregulation in the Tumor Microenvironment Contributes to Cancer Metastasis Liu W, Wang B, Zhou M, Liu D, Chen F, Zhao X, Lu Y. Antioxidants & Redox Signaling.2023;39(7-9). CrossRef
- Oxidative Stress in Melanoma: Beneficial Antioxidant and Pro-Oxidant Therapeutic Strategies Becker AL , Indra AK . Cancers.2023;15(11). CrossRef
- Oxidative stress and antioxidant defenses in biology: Springer Science & Business Media Ahmad S. 2012.
- The role of antioxidants in the chemistry of oxidative stress: A review Pisoschi AM , Pop A . European Journal of Medicinal Chemistry.2015;97. CrossRef
- Evaluation of LH, FSH, oestradiol, prolactin and tumour markers CEA and CA-125 in sera of Iraqi patients with endometrial cancer Dawood Y, Mahdi M, Jumaa A, Saad R, Khadim R. Scripta Medica.2024;55(4). CrossRef
- The Role of Natural Antioxidants Against Reactive Oxygen Species Produced by Cadmium Toxicity: A Review Unsal V, Dalkiran T, Çiçek M, Kölükçü E. Advanced Pharmaceutical Bulletin.2020;10(2). CrossRef
- Inflammation-associated genomic instability in cancer Pua KH , Chew CL , Lane DP , Tergaonkar V. Genome Instability & Disease.2020;1(1). CrossRef
- ROS: Basic Concepts, Sources, Cellular Signaling, and its Implications in Aging Pathways De Almeida AJPO , De Oliveira JCPL , Da Silva Pontes LV , De Souza Júnior JF , Gonçalves TAF , Dantas SH , De Almeida Feitosa MS , et al . Oxidative Medicine and Cellular Longevity.2022;2022(1). CrossRef
- Evaluation of quality of life for women with breast cancer Khalifa M, Ghadhban A, Hade I, Ali M. Scripta Medica.2024;55(1). CrossRef
- Interplay Between Redox Homeostasis and Oxidative Stress in the Perspective of Ovarian and Cervical Cancer Immunopathogenesis. Handbook of Oxidative Stress in Cancer: Mechanistic Aspects Kumar S, Mulchandani V, Banerjee A, Das Sarma J. 2020;:1-18.
- HPV: Molecular pathways and targets Gupta S, Kumar P, Das BC . Current Problems in Cancer.2018;42(2). CrossRef
- Biological role of 8-oxo-2'-deoxyguanosine Marmiy NV , Esipov DS . Moscow University Biological Sciences Bulletin.2015;70(4). CrossRef
- The Drivers, Mechanisms, and Consequences of Genome Instability in HPV-Driven Cancers Porter VL , Marra MA . Cancers.2022;14(19). CrossRef
- Oxidative Stress and Age-Related Tumors Di Carlo E, Sorrentino C. Antioxidants.2024;13(9). CrossRef
- New Insights in the Pathogenesis of HPV Infection and the Associated Carcinogenic Processes: The Role of Chronic Inflammation and Oxidative Stress Georgescu SR , Mitran CI , Mitran MI , Caruntu C, Sarbu MI , Matei C, Nicolae I, Tocut SM , Popa MI , Tampa M. Journal of Immunology Research.2018;2018. CrossRef
- Link between chronic inflammation and human papillomavirus-induced carcinogenesis (Review) Fernandes JV , De Medeiros Fernandes TAA , De Azevedo JCV , Cobucci RNO , De Carvalho MGF , Andrade VS , De Araújo JMG . Oncology Letters.2015;9(3). CrossRef
- Risk factors for cervical cancer in Iraqi women Abdulla KN , Skheel Aljebor HD , Mohson KI , Sabah Rasoul N, Mahdi MA . Libri Oncologici Croatian Journal of Oncology.2024;51(2-3). CrossRef
- Inflammation, cytokines, the IL-17/IL-6/STAT3/NF-κB axis, and tumorigenesis Zhou SF , Chen XW . Drug Design, Development and Therapy.2015. CrossRef
- Role of PI3K-AKT-mTOR Pathway as a Pro-Survival Signaling and Resistance-Mediating Mechanism to Therapy of Prostate Cancer Pungsrinont T, Kallenbach J, Baniahmad A. International Journal of Molecular Sciences.2021;22(20). CrossRef
- Oxidative stress-mediated epigenetic remodeling, metastatic progression and cell signaling in cancer Phull AR , Arain SQ , Majid A, Fatima H, Ahmed M, Kim SJ . Oncologie.2024;26(4). CrossRef
- Oxidative stress regulation and related metabolic pathways in epithelial–mesenchymal transition of breast cancer stem cells Farahzadi R, Valipour B, Fathi E, Pirmoradi S, Molavi O, Montazersaheb S, Sanaat Z. Stem Cell Research & Therapy.2023;14(1). CrossRef
- Tumor Hypoxia Regulates Immune Escape/Invasion: Influence on Angiogenesis and Potential Impact of Hypoxic Biomarkers on Cancer Therapies Abou Khouzam R, Brodaczewska K, Filipiak A, Zeinelabdin NA , Buart S, Szczylik C, Kieda C, Chouaib S. Frontiers in Immunology.2021;11. CrossRef
- Redox signaling-mediated tumor extracellular matrix remodeling: pleiotropic regulatory mechanisms Liu G, Li B, Qin S, Nice EC , Yang J, Yang L, Huang C. Cellular Oncology.2024;47(2). CrossRef
- Clinical Investigation of IL-31, TOS and GSH in the Sera of Gastric Cancer Females Patients In Iraq Mahdi M, Jumaa A, Dawood Y. Asian Pacific Journal of Cancer Prevention.2025;26(2). CrossRef
- NF-κB signaling in therapy resistance of breast cancer: Mechanisms, approaches, and challenges Guo Q, Jin Y, Lin M, Zeng C, Zhang J. Life Sciences.2024;348. CrossRef
- Reactive Oxygen Species, Antioxidant Responses and Implications from a Microbial Modulation Perspective Zandi P, Schnug E. Biology.2022;11(2). CrossRef
- First line defence antioxidants-superoxide dismutase (SOD), catalase (CAT) and glutathione peroxidase (GPX): Their fundamental role in the entire antioxidant defence grid Ighodaro OM , Akinloye OA . Alexandria Journal of Medicine.2018;54(4). CrossRef
- Bartosz G. Superoxide dismutases and catalase. Reactions, Processes: Oxidants and Antioxidant Defense Systems. 2004:109-49. .
- The glutathione peroxidases: Arthur JR . Cellular and Molecular Life Sciences.2001;57(13). CrossRef
- NRF2, a Transcription Factor for Stress Response and Beyond He F, Ru X, Wen T. International Journal of Molecular Sciences.2020;21(13). CrossRef
- Genetic Modulation of HPV Infection and Cervical Lesions: Role of Oxidative Stress-Related Genes Inácio Â, Aguiar L, Rodrigues B, Pires P, Ferreira J, Matos A, Mendonça I, et al . Antioxidants.2023;12(10). CrossRef
- Role of Nrf2/HO-1 system in development, oxidative stress response and diseases: an evolutionarily conserved mechanism Loboda A, Damulewicz M, Pyza E, Jozkowicz A, Dulak J. Cellular and Molecular Life Sciences.2016;73(17). CrossRef
- Emerging paradigms: unmasking the role of oxidative stress in HPV-induced carcinogenesis Letafati A, Taghiabadi Z, Zafarian N, Tajdini R, Mondeali M, Aboofazeli A, Chichiarelli S, Saso L, Jazayeri SM . Infectious Agents and Cancer.2024;19(1). CrossRef
- Peripheral blood immune cell parameters in patients with high-grade squamous intraepithelial lesion (HSIL) and cervical cancer and their clinical value: a retrospective study Wang L, Dong Y. PeerJ.2024;12. CrossRef
- Effects of Antioxidant Gene Overexpression on Stress Resistance and Malignization In Vitro and In Vivo: A Review Tavleeva MM , Belykh ES , Rybak AV , Rasova EE , Chernykh AA , Ismailov ZB , Velegzhaninov IO . Antioxidants.2022;11(12). CrossRef
- Dual roles of NRF2 in tumor prevention and progression: Possible implications in cancer treatment Moon EJ , Giaccia A. Free Radical Biology and Medicine.2015;79. CrossRef
- Oxidative stress in cervical cancer pathogenesis and resistance to therapy Ebrahimi S, Soltani A, Hashemy SI . Journal of Cellular Biochemistry.2019;120(5). CrossRef
- ROS, redox regulation, and anticancer therapy. Redox regulation and therapeutic approaches in cancer: Springer; 2023. p. 311-409. Bansal MP . .
- Role of Natural Antioxidants in Cancer Alsulami FJ , Shaheed SU . Nutrition and Dietary Interventions in Cancer.2024;191.
- Curcumin as a multifaceted compound against human papilloma virus infection and cervical cancers: A review of chemistry, cellular, molecular, and preclinical features Teymouri M, Pirro M, Johnston TP , Sahebkar A. BioFactors.2017;43(3). CrossRef
- Mechanisms and Applications of the Anti-cancer Effect of Pharmacological Ascorbic Acid in Cervical Cancer Cells Wu TM , Liu ST , Chen SY , Chen GS , Wu CC , Huang SM . Frontiers in Oncology.2020;10. CrossRef
- ROS-modulated therapeutic approaches in cancer treatment Raza MH , Siraj S, Arshad A, Waheed U, Aldakheel F, Alduraywish S, Arshad M. Journal of Cancer Research and Clinical Oncology.2017;143(9). CrossRef
- Emerging Roles of the Copper–CTR1 Axis in Tumorigenesis Su Y, Zhang X, Li S, Xie W, Guo J. Molecular Cancer Research.2022;20(9). CrossRef
- A Comprehensive Overview on Chemotherapy-Induced Cardiotoxicity: Insights into the Underlying Inflammatory and Oxidative Mechanisms Nagy A, Börzsei D, Hoffmann A, Török S, Veszelka M, Almási N, Varga C, Szabó R. Cardiovascular Drugs and Therapy.2025;39(5). CrossRef
- Photodynamic Therapy Review: Principles, Photosensitizers, Applications, and Future Directions Correia JH , Rodrigues JA , Pimenta S, Dong T, Yang Z. Pharmaceutics.2021;13(9). CrossRef
- Clinical treatment of intra-epithelia cervical neoplasia with photodynamic therapy Vendette ACF A, Piva HL , Muehlmann LA , De Souza DA , Tedesco AC , Azevedo RB . International Journal of Hyperthermia.2020;37(3). CrossRef
- Oxidative stress: therapeutic approaches for cervical cancer treatment Silva GAL , Nunes RAL , Morale MG , Boccardo E, Aguayo F, Termini L. Clinics.2018;73. CrossRef
- Antioxidant Therapy: Current Status and Future Prospects Firuzi O, Miri R, Tavakkoli M, Saso L. Current Medicinal Chemistry.2011;18(25). CrossRef
- Oxidative Stress and Antioxidants in Carcinogenesis and Integrative Therapy of Cancer Milkovic L, Siems W, Siems R, Zarkovic N. Current Pharmaceutical Design.2014;20(42). CrossRef
- Anti-oxidants as therapeutic agents for oxidative stress associated pathologies: future challenges and opportunities Nandha SR , Checker R, Patwardhan RS , Sharma D, Sandur SK . Free Radical Research.2025;59(1). CrossRef
- Current trends in triblock copolymer-based multifunctional nanotheranostics for cancer treatment Rahmanian M, Oroojalian F, Kesharwani P, Sahebkar A. Journal of Drug Delivery Science and Technology.2024;99. CrossRef
- Recent trends in the application of nanoparticles in cancer therapy: The involvement of oxidative stress Sanati M, Afshari AR , Kesharwani P, Sukhorukov VN , Sahebkar Amirhossein. Journal of Controlled Release.2022;348. CrossRef
- Biomarkers of Response and Resistance to DNA Repair Targeted Therapies Stover EH , Konstantinopoulos PA , Matulonis UA , Swisher EM . Clinical Cancer Research.2016;22(23). CrossRef
- Circulating lipid peroxidation and antioxidant status in cervical cancer patients: a case-control study Manju V, Kalaivani Sailaja J, Nalini N. Clinical Biochemistry.2002;35(8). CrossRef
- Novel therapeutic approaches targeting oxidative stress in breast and lung cancer Kaushik P, Kaushik M, Parvez S. Novel Therapeutic Approaches Targeting Oxidative Stress.2022.
- Combination Cancer Therapy with Immune Checkpoint Blockade: Mechanisms and Strategies Patel SA , Minn AJ . Immunity.2018;48(3). CrossRef
- The Emerging Role of Salivary Oxidative Stress Biomarkers as Prognostic Markers of Periodontitis: New Insights for a Personalized Approach in Dentistry Viglianisi G, Tartaglia GM , Santonocito S, Amato M, Polizzi A, Mascitti M, Isola G. Journal of Personalized Medicine.2023;13(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2026
Author Details