Surgical Outcomes and Prognostic Factors in Cervical Cancer: A 12 year, Single-center Experience
Download
Abstract
Objective: This study was conducted to evaluate the surgical outcomes of patients with early-stage cervical cancer and to identify clinicopathological factors that may predict a 5-year disease-free survival of patients who are treated with modified or radical hysterectomies and pelvic lymphadenectomy.
Methods: The record of 146 patients with early-stage invasive cervical carcinoma who had been treated at the HRH Princess Maha Chakri Sirindhorn Medical Center, in the period between January 2003 and December 2014 were retrospectively reviewed. Clinical and pathological variables that include age, wait time to surgery, stage of cancer, pelvic nodule status, lymphovascular space invasion, histology, depth of invasion, tumor grade, surgical margin status, parametrium involvement, and tumor size were recorded. The Kaplan-Meier statistical method was used for the calculation for the 5-year disease-free survival and the 5-year overall survival. The Log-rank test and Cox regression analysis were used to assess the significant factors relating to recurrence.
Result: A Large population in this study was in Stage IB1 (62%). The most common histology obtained was of squamous cell carcinoma (68%). Approximately 77% of the patients underwent either a modified or radical hysterectomy and 25% had received adjuvant treatment. The median time of patient follows up was 60 months. The estimated 5-year disease-free survival of the patients with early-stage cervical cancer was 84%. Recurrent disease occurred in 14% of the patients and the majority of these (71%) were localized metastases. Stage, nodular status and tumor size were significant as poor prognostic factors resulting from the univariate analysis study. However, there were no statistically significant associations between these factors and the 5-year DFS on multivariable analysis.
Conclusion: Early stage cervical cancer patients treated at our institute had favorable outcomes. The significant prognostic factors for disease-free survival were the stage, nodular status, and tumor size.
Introduction
Cervical cancer is the fourth most common cancer in women, and the seventh overall, with an estimated 528,000 new cases diagnosed in 2012 [1]. A Large majority (approximately 85%) occurs in the lower developed regions and the mortality rate is near 50%. The age-standardized incidence rates of cervical cancer and death rate in Thailand are 17.8 and 12.2 per 100,000 women per year, respectively [2]. Although the incidence rate has been decreasing, the mortality rate has not declined significantly.
Early stage cervical cancer is defined as the disease limited to the uterus or, as referred to by the International Federation of Gynecology and Obstetrics (FIGO) as stage IA or IB1 disease. Treatment options for these women include definitive surgery or radiation therapy with or without chemotherapy. The choice of treatment depends on the tumor stage and the patient-defined factors. In some selected cases of patients with IIa disease, this may also be treated with radical surgery. A comparison of surgery with radiation has resulted in similar survival rates for both treatment modalities. We prefer radical surgery over primary radiation. Surgery has the advantages of providing a more accurate evaluation of the extent of the disease, preserves the ovarian function, takes less treatment course time and provides an easier method to correct complications. The focus of this article is the patients with cervical cancer stages Ia to IIa, treated by primary surgery at the HRH Princess Maha Chakri Sirindhorm Medical Center. Our hospital has been fully functioning since 2003. We have reported on our long experience over the past 12 years (2003-2014).
This study was conducted to evaluate the effectiveness of surgical treatment in women with early-stage cervical cancer and to determine the factors affecting a disease-free survival and the recurrent rates of patients with early-stage cervical cancer who had been treated at our institution over a period of 12 years.
Materials and Methods
This is a retrospective analysis of the patients with cervical cancer who were treated surgically between January 2003 and December 2014 at the HRH Princess Maha Chakri Sirindhorm Medical Center, NakhonNayok, Thailand. The patients’ data, stage of disease, pathological findings, type of surgery, complications and adjuvant treatments were obtained from clinical charts, operative notes and pathological reports as provided by the Hospital Information System (HIS) of our hospital. After primary surgical treatment, the pathological findings were evaluated to assess the risk of recurrence for all patients. The women with moderate or high risk were referred to Mahavajiralongkorn Cancer Center in order to receive the adjuvant treatment, with or without chemotherapy. After completing the treatment program, the patients returned for follow up with the gynecological oncologists at our hospital every three months for the first two years, every six months up to five years and annually thereafter. We followed the status of patients by telephone in cases where they missed their appointments. The recent status of patients was updated by searching the data from the civil registration database at the district office. The primary outcome was the 5-year, disease-free survival (DFS) which is defined as the period from the date of completion of the initial therapy to the date of a recurrence diagnosis or the date of the last follow-up. Secondary outcomes were also investigated to consider some effects which have been associated with a recurrent disease in the patients treated with type II, III hysterectomies or radical trachelectomy. This study was approved by the Research Ethics Committee at the Faculty of Medicine, Srinakarinwirot University (SWU EC 308/54 E).
Statistical analysis was performed using the SPSS program (version 23.0, SPSS, Inc.). The Kaplan-Meier statistical method was used for the calculation of DFS and statistical significance was analyzed by the log-rank test. A multivariate Cox proportional hazards analysis with selected variables was used to determine the significantly important factors for recurrence. We considered the results to be statistically significant with the P-value <0.05.
Results
The clinicopathological variables of the patients are summarized in Tabl 1. The mean age of the patients was 45 years, range 25-75 years. Most of the patients were in the age range between 40-49 years. Of the patients, 70-75% of them was married and had at least one child. Of 146 patients, 18.5%, 4%, 62.3%, 11% and 4.1% were diagnosed in the stages of IA1, IA2, IB1, IB2 and IIA, respectively. Only 28% received primary surgery within 30 days following the diagnosis. However, approximately 20% of patients with stage IB1 – IIA were treated with a single dose of cisplatin prior to a radical hysterectomy done within 45 days. A hundred patients (68%) had histology that identified squamous cell carcinoma.
| Patient characteristics | Outcome (%) | |
| Age group (years old) | 25-39 | 48 (32.9) |
| 40-49 | 50 (34.2) | |
| 50-59 | 32 (21.9) | |
| 60-79 | 16 (11) | |
| Marital status | Single | 16 (11) |
| Married | 11 (75.3) | |
| Divorced | 6 (4.1) | |
| Widowed | 7 (4.8) | |
| Separate | 7 (4.8) | |
| Parity | 0 | 10 (6.8) |
| 1 to 3 | 97 (66.4) | |
| >3 | 31 (21) | |
| no data | 8 (5.5) | |
| The interval between diagnosis to surgery (days) | < 30 | 41 (28) |
| 30-45 | 49 (33.6) | |
| >45 | 56 (38.4) | |
| Stage of disease | IA1 | 27 (18.5) |
| IA2 | 6 (4) | |
| IB1 | 91 (62.3) | |
| IB2 | 16 (11) | |
| IIA1 | 6 (4.1) | |
| histological type | SCC | 100 (68) |
| Adenocarcinoma | 35 (24) | |
| Adenosquamous | 4 (2.7) | |
| Others | 7 (4.8) | |
| Tumor grade | well diff | 26 (17.8) |
| mod diff | 28 (19.2) | |
| poorly diff | 9 (6.2) | |
| not known | 83 (56.8) | |
| Tumor diameter | <1 cm | 64 (43.8) |
| 1 to 2 cm | 26 (17.8) | |
| 2 to <4cm | 35 (24) | |
| > 4cm | 21 (14.4) | |
| Type II, III and Trachelectomy specimens (N112) | ||
| Positive Pelvic node | 11 (7.5) | |
| Positive LVSI | 24 (21.4) | |
| Parametrium positive | 4 (3.5) | |
| Vaginal margin involvement | 7 (6.2) | |
| Deep Stromal invasion (inner one third) | 63 (56) | |
| Ovarian metastasis | 2 (1.7) |
As taken from Tabl 2, the operation consists of type I hysterectomy for 30 cases (20.5%), type II and III in 111 cases (76%), aborted hysterectomy due to pelvic node metastases for 4 cases (2.7%) and Radical Trachelectomy in 1 case (0.68%). All patients who had been diagnosed with stage IA2 or greater and stage IA1 with lymphovascular space invasion (LVSI) underwent bilateral pelvic lymphadenopathy with a mean number of 20 nodes removed (10.7 nodes from the right side and 9.6 nodes from the left) and 7.5% of these cases had pelvic node metastases. From the type II and type III hysterectomies, 111 specimens and 1 case with the procedure of a radical trachelectomy, 11(9.8%) had shown positive pelvic nodes, 24 (21.4%) had exhibited lymphovascular involvement, 4 (3.5%) had parametrial involvement, 7 (6.2%) had vaginal involvement and 2 (1.7%) had shown ovarian metastasis. Deep stromal invasion (to the outer third) was present in 63 cases (56%). Type II and III hysterectomies had longer operative times, longer hospital stays and had shown more blood loss than those with type I hysterectomies. Regarding the complications, 17 (11.6%) patients experienced massive intra-operative blood loss (≥1,000 ml), 2 (1.3%) had accidentally torn ureters and bladders and 2 (1.3%) had developed thromboembolism. Significant, persistent lymphocytes occurred in 5 cases (3.4%) and long-term bladder dysfunction was found in 2 cases (1.3%). One-third of the women (25%) received adjuvant treatments. With a median follow up period of 60 months, tumor recurrence occurred in 21 cases (14.3%): local metastasis in 15 cases (71.4%) and distance metastasis was seen in 6 cases (28.5%). Almost all such patients (90%) developed their recurrence within the first two years. At the time of analyses, 115 cases (78%) were alive and well, 16 were dead of the disease and 4 were alive with the disease. Three-year and five-year disease-free survivals were 87% and 84%, respectively.
| Variable | Outcome | |
| Operation | Type I hysterectomy | 30 (20.5%) |
| Type II/III hysterectomy | 111 (76.2%) | |
| Abort hysterectomy | 4 (2.7%) | |
| Trachelectomy | 1 (0.68%) | |
| Pelvic Lymphadenectomy | 120 (82.2%) | |
| Number of node removed, Mean (SD) | Total | 20.2 (7.1) |
| Right side | 11.3 (9.3) | |
| Left side | 9.5 (3.8) | |
| Operative time (min) Mean (SD) | Type I hysterectomy | 109 (36) |
| Type II/III hysterectomy | 206 (63) | |
| Blood loss (ml) Mean (SD) | Type I hysterectomy | 351 (36) |
| Type II/III hysterectomy | 870 (731) | |
| hospital stay (days) Mean (SD) | Type I hysterectomy | 5.5 (3.5) |
| Type II/III hysterectomy | 10 (1.4) | |
| Complication | none | 118 (80.8%) |
| excessive blood loss | 17 (11.6%) | |
| urinary tract injury | 2 (1.3%) | |
| thromboembolism | 2 (1.3%) | |
| persistent lymphocyte | 5 (3.4%) | |
| persistent bIadder dysfunction | 2 (1.3%) | |
| Adjuvant treatment | none | 110 (75.3%) |
| radiation | 15 (10.3%) | |
| chemotherapy | 12 (8.2%) | |
| chemoradiation | 9 (6.1%) | |
| Recurrent disease | 21 (14.3%) | |
| Mean Disease Free Interval (months) (SD,min-max) | 9 (16,2-66) | |
| Site of recurrent | Local | 15 (71.4%) |
| Distance | 6 (28.5%) | |
| Status of patient | Alive NOS | 33 (22.8%) |
| Alive NED | 92(63.4%) | |
| Alive with disease | 4(2.8%) | |
| Dead | 16(11%) | |
| Cause of dead | Cervical cancer | 3 |
| Intercurrent disease | 2 | |
| Unknown cause | 11 |
a)Outcomes are presented as numbers (percentage). Exceptions; the number of nodes, operative time, blood loss and hospital stay presented as means (SD)
(Fig 1) shows the Kaplan-Meier Survival Curve of the cumulative survival up until 12 years post-diagnosis. The survival probability continued to stabilize beyond the five to the six-year period following the diagnosis. The log-rank test was used to determine the impact of various clinicopathologic factors on the disease-free survival rates in early cervical cancer patients undergoing modified radical or radical hysterectomies. Stage, node status and tumor size were found to have an impact on recurrence in the initial analysis.
Figure 1 : The Kaplan-Meier Probability Overall Survival (A) and Disease-free Survival (B) of Women with Early Stage Cervical Cancer
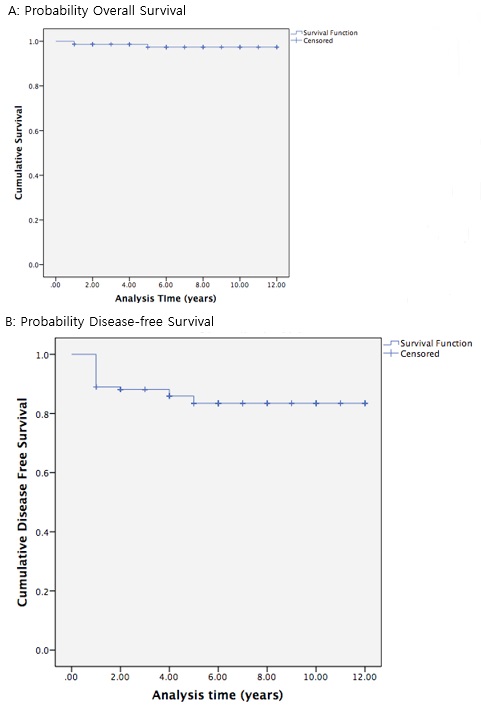
The 5-year DFS was 96%, 88%, 56% and 50% for patients with stage IA,IB1,IB2 and IIA1, respectively (P<0.05). The 5-year DFS was 85% for the patients without pelvic node metastases. Contrasting with 41% for those with pelvic node metastases (P= 0.01). The 5-year DFS was 90-93% for the patients with lesions less than 2 cm, 74% for those with lesions 2-4cm and only 64% for those with lesions Larger than 4cm. However, from multivariate analysis, there is no independent prognostic factor for the 5-year disease-free survival rates (Tabl 3).
| Variable | 5-years DFS | 10-years DFS | P Value* | P Value† |
| Age (years old) | 0.73 | 0.40 | ||
| 25-39 | 69% | 69% | ||
| 40-49 | 86% | 83% | ||
| 50-59 | 81% | 81% | ||
| 60-79 | 84% | 69% | ||
| Waiting time (days) | 0.18 | 0.43 | ||
| <30 | 78% | 78% | ||
| 30-45 | 79% | 74% | ||
| >45 | 93% | 86% | ||
| Stage | <0.05 | 0.14 | ||
| Ia | 96% | 96% | ||
| IB1 | 88% | 88% | ||
| Ib2 | 56% | 42% | ||
| IIa1 | 50% | 25% | ||
| Pelvic node | 0.01 | 0.20 | ||
| Negative | 85% | 81% | ||
| Positive | 41% | 41% | ||
| LVSI | 0.70 | 0.70 | ||
| Negative | 85% | 81% | ||
| Positive | 79% | 79% | ||
| Histology | 0.12 | 0.25 | ||
| SCC | 86% | 83% | ||
| Adenocarcinoma | 86% | 78% | ||
| Adenosquamous | 75% | 75% | ||
| Others | 50% | 50% | ||
| Depth | 0.50 | 0.92 | ||
| inner third | 81% | 78% | ||
| outer third | 100% | 75% | ||
| Parametrium | 0.59 | 0.98 | ||
| Negative | 82% | 78% | ||
| Positive | 67% | 67% | ||
| Tumor grade | 0.74 | 0.86 | ||
| Grade 1 | 86% | 76% | ||
| Grade 2 | 77% | 67% | ||
| Grade 3 | 71% | 71% | ||
| Margin | 0.84 | 0.98 | ||
| Negative | 84% | 81% | ||
| Positive | 33% | - | ||
| Tumor size | <0.05 | 0.16 | ||
| <1cm | 93% | 93% | ||
| 1-2cm | 90% | 90% | ||
| 2-4cm | 74% | 68% | ||
| >4 | 64% | 49% |
a) DFS: Disease-free survival
b) Obtained from log-rank (Mantel-Cox) test. †Obtained from Cox-Proportional hazards model
Discussion
A total of 146 patients with early-stage cervical cancer had visited the HRH Princess Maha Chakri Sirindhorm Medical Center over the last 12 years. The number of cases per year had been rising as our institute has become the referral center since 2013. The mean age of the patients was 45 years with the majority of patients in the age group of 40-49 years which correlates with the ages for HPV-associated cervical cancer [2]. Most of the patients were married and had at least one child. The general characteristics are consistent with many studies that had been done in other regions of Thailand [2, 3, 4, 5].
The 5-year DFS of localized, cervical cancer found in this study was 84% which is consistent with other centers in both Thailand and other countries [6, 7, 8]. And these results are better than the survival seen from a study of the International Agency for Research on Cancer (IARC) which was conducted in Thailand, the Philippines, India and Costa Rica [9]. However, the survival for each FIGO stage was slightly lower than that reported in the Chiang Mai province, Thailand [3, 4, 10]. Based on univariate analysis, the stage, pelvic node involvement, and tumor size significantly correlate with DFS. Some authors have also reported that the stage has an effect on survival from the disease [10, 11, 12]. One study had revealed that the survival rate correlates more consistently with tumor volume than clinical or histological stage [13]. In this study, the 5-year DFS was 96%, 88%, 56% and 50% for patients with stage IA, IB1, IB2, and IIA1, respectively (P<0.05). Regard to pelvic node status, the 5-year DFS was 85% for the patients without pelvic node metastases, in contrast to 41% for those with pelvic node metastases (P 0.01). In our protocol, patients with pelvic node metastases received postoperative adjuvant radiation and chemotherapy. Adjuvant treatment may not have improved the prognosis of these patients. There were 75% of the patients with positive pelvic nodes and had lesions larger than 2 cm. Patients with pelvic node metastases and tumor size larger than 2 cm had the worst prognosis: the 5-year DFS was 33% in contrast to 89% of those without pelvic node involvement and lesions smaller than 2 cm. These factors may be interrelated. Nevertheless, the multivariate analysis did not reflect the true prognoses.
The DFS was not significantly different for the patients’ age, wait time to surgery, LVSI, histology, depth of invasion, parametrial invasion, the grade of the tumor and disease status at the surgical margin. Some of these factors have shown a correlation with the prognosis of the disease in previous studies [7, 12, 14, 15, 16, 17]. This difference may have resulted from the wide variation between individual studies. These variations resulted primarily in the number of samples, stage of the disease, the treatment modality, and the accessibility to follow-up. There is no evidence that shows that a longer waiting time affects the overall survival (OS) in the first 5 years [11, 18, 19]. One study has shown that a wait time to surgery longer than 8 weeks leads to worse OS after 5 years. However, the information on the cause of deaths in this study was not available [5]. The presence of LVSI was not predictive of a subsequent recurrence of early-stage cervical cancer in many studies [3, 11, 20]. One literature review showed that only three of 25 articles (12%) identified LVSI as an independent risk factor [21]. Some studies have determined that LVSI is an independent prognosticating factor [6, 10, 17]. From the previous studies done in Thailand, the incidence of positive LVSI in stage IA2, IB, and IIA were 10.3, 52.6 and 38.6% respectively [3, 4, 10]. In our study, most patients were in stage IB and only 21.4% of all patients had positive LVSI. It is likely that this study did not have adequate power to detect the association of LVSI and treatment outcomes.
Reports have conflicting views about the effects of the histological types on the outcomes. Several population-based and retrospective studies have shown worse outcomes for patients with adenocarcinoma with an increased distant metastasis when compared with those with squamous cell histology [15, 22, 23]. One report showed that an adenosquamous cell type behaved more aggressively than the pure squamous counterparts [24]. Farley JH reported that adenosquamous histology predicted a poor outcome for patients in advanced stages, but not early-stage cervical carcinoma [14]. In our study, women with adenosquamous cell carcinoma histology were more likely to develop the recurrent disease but the results are not statistically significant.
Although there are a lot of studies regarding cervical cancer in Thailand, most of those were conducted in regions in which their incidences and histological distributions differed. The strengths of this study were the inclusion of patients who were treated at a single institution with long-term follow up. All pathological specimens were examined by experienced pathologists at our institute. We also updated the Latest status of the patients from the records of civil registration to minimize the loss to follow up rates. However, the design of this study is retrospective and some important data was not available and thus, had not been completed. As there is no Radiotherapy Unit in our hospital, we referred some patients to a nearby hospital for the adjuvant treatment. The data regarding the time to access radiation delivery, compliance and complications of adjuvant therapies were not included in this study. These may affect the results of the prognosis of the disease. In conclusion, the five-year DFS for early-stage cervical cancer seen at our institute was 84% which is comparable to the results seen in previous studies. Stage, LN status and tumor size were important prognostic factors as assessed from univariate analysis but no statistically significant effects were seen in the multivariate analysis. Further studies of survival rates with larger sample sizes are required to help confirm the results. However, our data can be used as the baseline epidemiologic data for our institute and provide the physicians with some guidance in counseling patients about the consideration of adjuvant therapy.
References
[1]. FerIay J SI, Ervik M, Dikshit R, Eser S, et al Cancer Incidence and Mortality Worldwide: IARC CancerBase. No. 11 [Internet]. GLOBOCAN. 2012;1.
[2]. Bruni L B-RL AG, Aldea M, et al. ICO Information Centre on HPV and Cancer (HPV Information Centre). Human Papillomavirus and Related Diseases in Thailand Summary Report. 2015:12-23.
[3]. Mahawerawat S, Charoenkwan K, Srisomboon J, Khunamornpong S, Suprasert P, Sae-Teng CT. Surgical outcomes of patients with stage IA2 cervical cancer treated with radical hysterectomy. Asian Pac J Cancer Prev. 2013;14(9):5375-8.
[4]. Hongladaromp W, Tantipalakorn C, Charoenkwan K, Srisomboon J. Locoregional spread and survival of stage IIA1 versus stage IIA2 cervical cancer. Asian Pac J Cancer Prev. 2014;15(2):887-90.
[5]. Nanthamongkolkul K, Hanprasertpong J. Longer waiting times for early-stage cervical cancer patients undergoing radical hysterectomy are associated with diminished long-term overall survival. Journal of Gynecologic Oncology. 2015;26(4):262-9.
[6]. Metindir J, Bilir G. Prognostic factors affecting disease-free survival in early-stage cervical cancer patients undergoing radical hysterectomy and pelvic-paraaortic lymphadenectomy. Eur J Gynaecol Oncol. 2007;28(1):28-32.
[7]. Ho CM, Chien TY, Huang SH, Wu CJ, Shih BY, Chang SC. Multivariate analysis of the prognostic factors and outcomes in early cervical cancer patients undergoing radical hysterectomy. Gynecol Oncol. 2004;93(2):458-64.
[8]. Chittithaworn S, Hanprasertpong J, Tungsinmunkong K, Geater A. Association between prognostic factors and disease-free survival of cervical cancer stage IB1 patients undergoing the radical hysterectomy. Asian Pacific journal of cancer prevention: APJCP. 2007;8(4):530-4.
[9]. Sankaranarayanan R. Cancer survival in Africa, Asia, the Caribbean and Central America. Introduction. IARC Sci Publ. 2011(162):1-5.
[10]. Srisomboon J, Kietpeerakool C, Suprasert P, Manopanya M, Siriaree S, Charoenkwan K, et al. Survival and prognostic factors comparing stage IB 1 versus stage IB 2 cervical cancer treated with primary radical hysterectomy. Asian Pacific journal of cancer prevention: APJCP. 2011;12(7):1753-6.
[11]. Perri T, Issakov G, Ben-Baruch G, Felder S, Beiner ME, Helpman L, et al. Effect of treatment delay on survival in patients with cervical cancer: a historical cohort study. Int J Gynecol Cancer. 2014;24(7):1326-32.
[12]. Suprasert P, Srisomboon J, Charoenkwan K, Siriaree S, Cheewakriangkrai C, Kietpeerakool C, et al. Twelve years experience with radical hysterectomy and pelvic lymphadenectomy in early stage cervical cancer. J Obstet Gynaecol. 2010;30(3):294-8.
[13]. Burghardt E, Baltzer J, Tulusan AH, Haas J. Results of surgical treatment of 1028 cervical cancers studied with volumetry. Cancer. 1992;70(3):648-55.
[14]. Farley JH, Hickey KW, Carlson JW, Rose GS, Kost ER, Harrison TA. Adenosquamous histology predicts a poor outcome for patients with advanced-stage, but not early-stage, cervical carcinoma. Cancer. 2003;97(9):2196-202.
[15]. Lee YY, Choi CH, Kim TJ, Lee JW, Kim BG, Lee JH, et al. A comparison of pure adenocarcinoma and squamous cell carcinoma of the cervix after radical hysterectomy in stage IB-IIA. Gynecol Oncol. 2011;120(3):439-43.
[16]. Biewenga P, van der Velden J, Mol BW, Stalpers LJ, Schilthuis MS, van der Steeg JW, et al. Prognostic model for survival in patients with early stage cervical cancer. Cancer. 2011;117(4):768-76.
[17]. Takeda N, Sakuragi N, Takeda M, Okamoto K, Kuwabara M, Negishi H, et al. Multivariate analysis of histopathologic prognostic factors for invasive cervical cancer treated with radical hysterectomy and systematic retroperitoneal lymphadenectomy. Acta Obstet Gynecol Scand. 2002;81(12):1144-51.
[18]. Umezu T, Shibata K, Kajiyama H, Yamamoto E, Mizuno M, Kikkawa F. Prognostic factors in stage IA-IIA cervical cancer patients treated surgically: does the waiting time to the operation affect survival? Archives of gynecology and obstetrics. 2012;285(2):493-7.
[19]. Yoshino K, Hosoi A, Osuga K, Enomoto T, Ueda Y, Sawada K, et al. Single-dose intra-arterial neoadjuvant chemotherapy while waiting for radical hysterectomy for stage IB-IIB cervical cancer. Molecular and clinical oncology. 2016;4(6):1068-72.
[20]. Marchiole P, Buenerd A, Benchaib M, Nezhat K, Dargent D, Mathevet P. Clinical significance of lymphovascular space involvement and lymph node micrometastases in early-stage cervical cancer: a retrospective case-control surgical-pathological study. Gynecologic oncology. 2005;97(3):727-32.
[21]. Creasman WT, Kohler MF. Is lymph-vascular space involvement an independent prognostic factor in early cervical cancer? Gynecologic oncology. 2004;92(2):525-9.
[22]. Park JY, Kim DY, Kim JH, Kim YM, Kim YT, Nam JH. Outcomes after radical hysterectomy in patients with early-stage adenocarcinoma of uterine cervix. Br J Cancer. 2010;102(12):1692-8.
[23]. Galic V, Herzog TJ, Lewin SN, Neugut AI, Burke WM, Lu YS, et al. Prognostic significance of adenocarcinoma histology in women with cervical cancer. Gynecol Oncol. 2012;125(2):287-91.
[24]. Bethwaite P, Yeong ML, Holloway L, Robson B, Duncan G, Lamb D. The prognosis of adenosquamous carcinomas of the uterine cervix. Br J Obstet Gynaecol. 1992;99(9):745-50.
License
Copyright
© ,
Author Details