Combination of Eleutherine bulbosa Extract and Tamoxifen Suppresses 7,12-dimethylbenz[a]anthracene (DMBA)-Induced Breast Cancer: In Vivo Evidence of Enhanced p53 Expression and Histopathological Improvement
Download
Abstract
Background: Eleutherine bulbosa (Dayak onion), a phytotherapeutic plant rich in flavonoids and polyphenols, exhibits potent anticancer activity. As monotherapy frequently leads to treatment resistance, combining Eleutherine bulbosa ethanolic extract with standard endocrine therapy such as tamoxifen offers a promising approach to overcome resistance and improve treatment outcomes in breast cancer. This study evaluated whether simultaneous administration improves p53 activation and restores mammary tissue architecture in a DMBA-induced breast cancer mouse model.
Materials and Methods: An in vivo post-test-only design was employed, involving 36 female BALB/c mice (n = 6 per group), including monotherapy, sequential combination (I3), and simultaneous combination (I4) regimens. Primary endpoints included histopathological evaluation of mammary tissue and quantification of p53 protein using Enzyme-Linked Immunosorbent Assay (ELISA) and Immunohistochemistry (IHC). Histological architecture was assessed by Hematoxylin and Eosin (HE) staining. Histology was performed on a single representative sample per group (n = 1), therefore interpreted descriptively.
Results: In vivo findings demonstrated that the simultaneous combination (I4) provided the most comprehensive therapeutic benefit. Group I4 showed the highest plasma p53 level (8.70 ± 0.30 pg/mL), which was significantly higher than that of the positive control (KP) but statistically comparable to tamoxifen monotherapy (I2, 8.09 ± 0.40 pg/mL). Similarly, the percentage of p53-positive cells detected by IHC did not differ significantly between I4 and I2. However, group I4 displayed the most pronounced morphological recovery, exhibiting the highest mean alveolar diameter (106.71 µm) and the lowest mean alveolar density (1.8/field), indicating the most favorable histological trend in reversing tissue damage compared to I2.
Conclusion: Simultaneous administration of Eleutherine bulbosa extract with tamoxifen provided the most favorable morphological trend compared with monotherapies, likely through localized, tissue-level actions rather than systemic p53 elevation.
Introduction
Cellular regulation plays a fundamental role in maintaining tissue homeostasis by identifying and eliminating abnormal, damaged, or mutated cells [1, 2]. When these regulatory mechanisms become impaired, abnormal cells may evade surveillance and continue to proliferate, ultimately leading to tumor formation.
Among the central components of this defense system is the p53 protein, widely known as the “guardian of the genome,” which coordinates multiple tumor-suppressive pathways to prevent malignant transformation [3, 4]. Reduced expression or compromised functionality of p53 is strongly associated with breast cancer development and diminished responsiveness to endocrine therapy [5, 6].
Breast cancer remains the most commonly diagnosed malignancy among women worldwide. According to GLOBOCAN 2022, it accounts for 11.6% of all global cancer cases and ranks as the fourth leading cause of cancer-related mortality (6.9%) [7]. In Indonesia, the disease presents a similarly concerning trend, with approximately 65,000 new cases reported annually, making it the most prevalent cancer among women [8]. These epidemiological patterns emphasize the urgent need for more effective therapeutic strategies capable of addressing not only disease progression but also treatment resistance, a major obstacle in hormone-based therapies. Approximately 75% of breast cancer cases express estrogen receptors (ER+), making them suitable targets for endocrine agents such as tamoxifen.
Tamoxifen, a widely used Selective Estrogen Receptor Modulator (SERM), inhibits tumor proliferation by competitively antagonizing estrogen receptor signaling in breast tissue. Despite its clinical effectiveness, its long-term therapeutic value is frequently diminished by the development of acquired resistance [6, 9]. A key factor underlying this resistance is p53 dysfunction, which weakens intrinsic tumor-suppressive mechanisms and enables cancer cells to activate alternative survival pathways [10]. Resistant tumors often exploit compensatory cascades, most notably the PI3K/Akt and NF-κB pathways, to bypass estrogen receptor blockade and maintain their proliferative capacity [6, 11]. Addressing this bypass phenomenon requires adjunct therapeutic strategies that can modulate central regulatory pathways, including p53.
Eleutherine bulbosa has gained attention as a potential complementary therapeutic agent due to its intrinsic tumor-suppressive effects and its ability, reported in previous experimental studies, to stabilize or modulate p53 activity. These attributes suggest that it may help counteract key signaling pathways associated with tamoxifen resistance, thereby improving treatment outcomes. Eleutherine bulbosa Urb., commonly referred to as Dayak onion, is an endemic medicinal plant from the Iridaceae family native to Borneo [12]. Its bulbs contain a rich profile of bioactive polyphenols and flavonoids that exhibit antioxidant, anti-inflammatory, and antidiabetic properties [13]. Prior in vitro studies have demonstrated its cytotoxic and antiproliferative activities, including effects linked to modulation of p53-related pathways [14, 15].
Ethnobotanical usage further supports i ts pharmacological relevance. For generations, the Dayak community has used Eleutherine bulbosa bulbs to treat a variety of illnesses, such as cancer, high blood pressure, stroke, diabetes, and stomach problems [16]. This long-standing traditional application provides a strong foundation for scientific validation and therapeutic development.
Given the complementary mechanisms of action of tamoxifen functioning as an estrogen receptor antagonist and Eleutherine bulbosa as a potential modulator of p53 stability, this study aimed to investigate whether their combined administration could enhance therapeutic efficacy in a DMBA-induced breast cancer model. We hypothesized that co-treatment would exert greater biological activity than monotherapy, reflected by enhanced p53 activation and improved mammary tissue histoarchitecture.
Materials and Methods
Research design
This experimental study adopted a post-test-only control group design to investigate the impact of Eleutherine bulbosa ethanol extract and tamoxifen on p53 protein expression and histopathological profiles in a DMBA-induced murine model of breast carcinogenesis.
Drugs and chemicals
Tamoxifen citrate (Tamofen®, 10 mg; PT Kimia Farma, Bandung, Indonesia) was prescribed and used as a hormonal therapy agent. 7,12-Dimethylbenz[a]anthracene (DMBA; ≥98% purity; Sigma-Aldrich, St. Louis, MO, USA) was obtained from the Healthy Animal Laboratory (Malang, Indonesia) and used as a carcinogenic inducer. Ethanol 96% (PT Brataco Chemical, Jakarta, Indonesia) served as the solvent for the maceration extraction of Eleutherine bulbosa.
Extract preparation
Eleutherine bulbosa (Dayak onion) tubers were collected in August 2024 from the Dayak region, Central Kalimantan, Indonesia. A total of 40 kg of fresh specimens were taxonomically identified at the Mulawarman Herbarium, Laboratory of Tropical Forest Ecology and Biodiversity Conservation, Faculty of Forestry, Mulawarman University, Samarinda. The plant identification (Letter No. 306/UN17.4.08/LL/2024, dated October 4, 2024, signed by Prof. Dr. Ir. Paulus Matius, M.Sc.) confirmed the species as Eleutherine bulbosa (Mill.) Urb., family Iridaceae. A voucher specimen (No. MUL-EB-2024) was deposited at the Mulawarman Herbarium for future reference.
The collected tubers were cleaned, thinly sliced, and dried at 40°C using a food dryer, then ground into a fine powder (simplicia). The total amount of dried simplicia obtained was 12 kg. Extraction was performed by maceration using 96% ethanol as the solvent. A total of 2 kg of dried simplicia was processed at a ratio of 1:20 b/v (weight of simplicia to solvent volume). The powdered material was soaked in 40 L of 96% ethanol for 48 hours. The resulting macerate was filtered and concentrated using a rotary evaporator (Heidolph Hei-VAP Core HL/G3) at 40°C. After solvent evaporation, a thick extract weighing 120 g was obtained, corresponding to a yield of 6.0% from the initial 2 kg of simplicia. The viscous extract was stored in an amber glass bottle at room temperature until further analysis.
Phytochemical analysis of extract
Phytochemical analysis of the Eleutherine bulbosa ethanol extract was conducted to identify its chemical constituents and quantify the levels of major bioactive compounds, with a particular focus on flavonoids and polyphenols due to their established biological relevance to anticancer activity. Qualitative phytochemical screening was performed using standard methods based on specific color reactions and precipitation tests [17] to detect key secondary metabolites, including flavonoids, alkaloids, tannins, saponins, terpenoids, and steroids.
Quantitative phytochemical analysis was carried out using colorimetric methods on a UV-Vis spectrophotometer (Shimadzu UV−1900, Japan). Total flavonoid content (TFC) was determined using the aluminum chloride (AlCl₃) colorimetric method [18]. With results estimated from a quercetin standard calibration curve and expressed as milligrams of quercetin equivalent per gram of dry extract (mg QE/g extract). Total polyphenol content (TPC) was quantified using the Folin-Ciocalteu method, with results calculated based on a gallic acid standard calibration curve and expressed as milligrams of gallic acid equivalent per gram of dry extract (mg GAE/g extract).
The extract was standardized through these spectrophotometric measurements to ensure batch consistency and experimental reproducibility. All analyses were performed in an accredited biochemistry laboratory.
Experimental animals
Thirty-six healthy female BALB/c mice (Mus musculus), aged 8–10 weeks and weighing 18–25 g, were used. Prior to experimentation, the animals were acclimatized for seven days under controlled environmental conditions (temperature 22–24°C; 12-hour light/dark cycle; and humidity of 50–60%) with ad libitum access to standard feed and water [19]. Only active and healthy mice showing no clinical signs of disease during the acclimatization period were included in the study. All experimental procedures were conducted in accordance with the ARRIVE guidelines and were approved by an independent animal ethics committee (Ethical Clearance No. 071/UN4.14.1/TP.01.02/2025).
To ensure data validity while strictly adhering to the 3R principles of animal ethics (Replacement, Refinement, Reduction), the sample size was set at n = 6 per group. This minimum number was determined based on published literature and preliminary studies, which demonstrated that n = 6 provides a statistical power of ≥80% to detect biologically significant differences between groups (p < 0.05).
Study design
A simple randomization procedure was applied to allocate the 36 mice into six groups (n = 6 per group): a negative control (KN), a positive control (KP), and four treatment groups (I1–I4). Random allocation was performed utilizing sealed envelopes prior to treatment initiation.
The six experimental groups were assigned as follows:
Negative Control (KN): Healthy mice without DMBA induction or any intervention.
Positive Control (KP): DMBA-induced mice receiving 0.5% CMC-Na solution as a vehicle control.
Intervention 1 (I1): DMBA-induced mice treated with Eleutherine bulbosa ethanol extract at 180 mg/kg body weight/day.
Intervention 2 (I2): DMBA-induced mice treated with tamoxifen at 5 mg/kg body weight/day, dissolved in 0.5% CMC-Na.
Intervention 3 (I3): DMBA-induced mice treated sequentially with Eleutherine bulbosa extract (180 mg/kg body weight/day), followed two hours later by tamoxifen (5 mg/kg body weight/day) (Sequential Combination).
Intervention 4 (I4): DMBA-induced mice treated with a simultaneous combination of Eleutherine bulbosa extract (180 mg/kg body weight/day) and tamoxifen (5 mg/kg body weight/day) dissolved/suspended in 0.5% CMC-Na (Simultaneous Combination).
All treatments were administered orally using a gavage (sonde) needle according to the specific dosing schedule for each group throughout the designated treatment period.
Cancer induction and treatment
Breast cancer was induced in female Balb/c mice through daily oral gavage of 7,12-dimethylbenz [a] anthracene (DMBA) at 50 mg/kg body weight for 42 consecutive days. This established protocol reliably produces mammary tumors that closely mimic human estrogen receptor-positive (ER⁺) breast cancer with p53 dysfunction. The initial average body weight of the mice was 20 g (0.02 kg). DMBA was dissolved in sesame oil to prepare a stable 1% (w/v) solution (10 mg/mL), which is routinely used for oral administration studies. Each mouse received a daily dose of 1 mg DMBA (50 mg/kg × 0.02 kg), delivered in 0.1 mL of the solution. Successful carcinogenesis was achieved in the positive control group (KP) and all treatment groups (I1-I4), as confirmed by clinical palpation of mammary nodules during the induction period and definitive histopathological examination at study termination. Thus, DMBA administration effectively modeled the initiation phase of breast carcinogenesis. Following the 42-day induction period, therapeutic interventions were administered orally via gavage for 14 days to groups I1–I4 [20, 21]. The extract from Eleutherine bulbosa was suspended in 0.5% carboxymethylcellulose sodium (CMC-Na), a safe and standard vehicle, and dosed at 180 mg/kg/day. This dose was selected based on prior in vivo efficacy studies and alignment with the no-observed-adverse-effect level (NOAEL) from sub-chronic toxicity assessment [22]. Tamoxifen was administered at 5 mg/kg/day, a well-established effective dose in ER⁺ murine models that approximates human-equivalent exposure, enabling evaluation of therapeutic response and potential resistance mechanisms.
Throughout the entire 56-day study (42 days of DMBA induction + 14 days of intervention), animal body weight and general behavior were monitored daily. Any signs of toxicity, including significant weight loss (>10%) or abnormal behavior, were documented. No treatment-related adverse effects were observed with either Eleutherine bulbosa extract or tamoxifen, confirming that both compounds were well tolerated at the administered doses.
On day 57 (i.e., 24 hours after the final intervention dose), mice were humanely euthanized in accordance with institutional ethical guidelines. Blood samples were collected via retro-orbital sinus puncture, and mammary gland tissues were excised for subsequent histopathological and immunohistochemical analyses.
Determination of p53 expression
At the end of the treatment period, mice were humanely euthanized by cervical dislocation under light ketamine–xylazine anesthesia (ketamine 50 mg/kg BW + xylazine 5 mg/kg BW, i.p.) in full compliance with institutional ethical guidelines. Blood was collected from the retro-orbital sinus into heparinized tubes, centrifuged at 1,500 × g for 10 min at 4°C, and the resulting plasma stored at −80°C until analysis. Plasma p53 concentrations were determined using a commercial mouse p53 sandwich ELISA kit (Primacu™ Mouse p53 ELISA Kit, Cat. No. BPE364) according to the manufacturer’s instructions. Absorbance was measured at 450 nm using a microplate reader (Bio-Rad iMark), and p53 levels (pg/mL) were calculated from a four-parameter logistic standard curve. Mammary gland tissue was excised, fixed in 10% neutral buffered formalin for 24–48 hours, routinely processed, and embedded in paraffin. Sections (4 µm thick) were cut, deparaffinized, and antigen retrieval was performed by heating in 10 mM citrate buffer (pH 6.0) for 20 min in a microwave oven. Endogenous peroxidase activity was blocked with 3% hydrogen peroxide for 10 min, and nonspecific binding was minimized by incubation with 5% normal goat serum for 30 min. Sections were incubated overnight at 4°C with rabbit monoclonal anti-p53 antibody (clone SP5, 1:100 dilution; Abcam, ab227651). Immunoreactivity was visualized using an HRP-conjugated streptavidin–biotin system (Dako REAL™ EnVision™) with 3,3′-diaminobenzidine (DAB) as chromogen, followed by counterstaining with Mayer’s haematoxylin.
Quantification of nuclear p53 expression was performed in a blinded manner. For each animal, five non-overlapping high-power fields (400× magnification, Nikon Eclipse type Ei microscope) were randomly selected from tumor-rich regions. Images were captured using an Optilab Microscope Camera attached to the microscope. In each field, at least 500 tumor cells were counted manually on the digital images. A nucleus was considered positive when distinct brown staining was observed, irrespective of staining intensity. The labelling percentage (LP) was calculated as:
LP (%) = (number of p53-positive tumor cell nuclei ÷ total number of tumor cell nuclei counted) × 100.
The mean LP from the five fields was taken as the value for that animal. All counts were performed independently by two observers, with inter-observer agreement exceeding 95%.
This combination of systemic (ELISA) and localized (IHC with a clearly defined, reproducible counting protocol) measurements gave a full picture of the p53 status in the DMBA-induced breast cancer model.
Histopathological examination of breast tissue
Mammary tissue samples were collected immediately after euthanasia and fixed in 10% neutral buffered formalin for at least 24 hours to preserve cellular architecture. The tissues were then processed using the paraffin embedding method, sectioned at 4-6 µm thickness with a manual microtome, and stained using the hematoxylin-eosin (H&E) technique for morphological evaluation. Stained sections were examined under an optical microscope (Nikon Eclipse type Ei) at 400× magnification. Observations were documented using a digital microscope camera connected to a computer. Measurements of mammary gland diameter and number of alveoli were taken from five different fields of view, and the results were averaged. The data were analyzed to assess changes in mammary tissue morphology in response to the treatments.
Histopathological evaluation and histomorphometric assessment were performed on one representative sample (n=1) per treatment group. This animal was selected based on the individual whose final plasma p53 level (ELISA) was closest to the group mean, ensuring the specimen biologically represented the group’s molecular response. Given the n=1 design for histological assessment, inferential statistical analysis was not feasible; therefore, findings are presented descriptively to illustrate morphological trends. To ensure objectivity, all evaluations were conducted systematically and blindly by an independent pathologist, following Bolon’s recommendations to minimize assessment bias.
Statistical analysis
Data were analyzed using SPSS version 26. Prior to hypothesis testing, the assumptions of normality and homogeneity of variance were evaluated using the Shapiro–Wilk test and Levene’s test, respectively. For data meeting these assumptions, differences between treatment groups were assessed using one-way analysis of variance (ANOVA), followed by Tukey’s post hoc test for pairwise comparisons. Quantitative results are presented as mean ± standard deviation (SD). Statistical significance was set at p < 0.05.
Results
Phytochemical characterization of extracts
Phytochemical analysis revealed that the ethanol extract of Eleutherine bulbosa contained flavonoids (0.9188%) and phenolics/polyphenols (1.5231%) (Table 1).
| Phytochemical Class | Qualitative Detection | Quantitative Content | Analytical Method |
| (% w/w) | |||
| Flavonoids | + | 0.9188 | Aluminium chloride colorimetric method |
| Polyphenols | + | 1.5231 | Folin–Ciocalteu method |
The qualitative and quantitative phytochemical screening confirmed the presence of flavonoids and polyphenols as the predominant bioactive constituents in the standardized ethanolic extract of Eleutherine bulbosa (Dayak onion) bulbs.
Effect of ethanol extract of Eleutherine bulbosa on p53 protein levels
The present study investigated the effects of ethanolic Eleutherine bulbosa extract, administered either alone or in combination with tamoxifen, on plasma p53 protein levels in a DMBA-induced breast cancer mouse model. One-way ANOVA demonstrated highly significant differences among the experimental groups (F (5,30) = 248.084; p < 0.001) (Table 2). Tukey’s HSD post-hoc test indicated that plasma p53 concentrations in all treatment groups (I1, I2, I3, and I4) as well as in the negative control (healthy mice, KN) were significantly higher than those observed in the positive control (DMBA-only, KP) (p < 0.001). Of particular note, no significant difference was found between tamoxifen monotherapy (I2: 8.09 ± 0.40 pg/mL) and the simultaneous combination regimen (I4: 8.70 ± 0.30 pg/mL) (p = 0.069); both groups belonged to the same homogeneous subset (Table 2).
| No | Treatment Group | n | p53 Protein Levels (pg/mL) Mean ± SD | Tukey HSD (p < 0.05) * |
| 1 | Negative control (Healthy, KN) | 6 | 9.80 ± 0.50 | a |
| 2 | Simultaneous combination (I4) | 6 | 8.70 ± 0.30 | b |
| 3 | Tamoxifen monotherapy (I2) | 6 | 8.09 ± 0.40 | b |
| 4 | Sequential combination (I3) | 6 | 6.93 ± 0.30 | c |
| 5 | Eleutherine bulbosa extract monotherapy (I1) | 6 | 5.04 ± 0.40 | d |
| 6 | Positive control (DMBA only, KP) | 6 | 3.62 ± 0.20 | e |
Footnote: • Means with different superscript letters in the same column are significantly different (One way ANOVA followed by Tukey’s HSD post-hoc test; F(5,30) = 248.084, p < 0.001). • Groups sharing the same letter are not significantly different (p > 0.05). Note: Tamoxifen monotherapy (I2) and simultaneous combination (I4) belong to the same homogeneous subset (p = 0.069).
These systemic findings were corroborated and validated by immunohistochemical (IHC) analysis of mammary tissue (Figure 1).
Figure 1. Immunohistochemical (IHC) Staining of p53 in DMBA-induced Mammary Tissue of BALB/c Mice (400×; scale bar = 100 µm). Brown nuclear staining indicates p53-positive cells (red arrows). Panels: (A) Normal Control (KN); (B) Positive Control (KP); (C) Eleutherine bulbosa monotherapy (I1); (D) Tamoxifen monotherapy (I2); (E) Sequential combination (I3); (F) Simultaneous combination (I4).
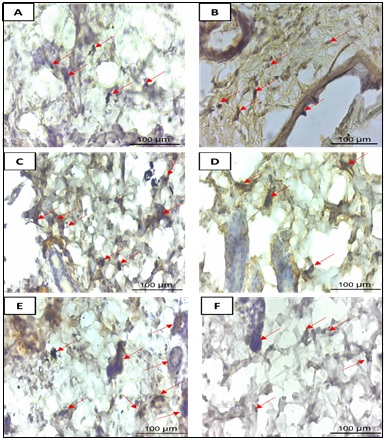
Quantitative assessment of nuclear p53 expression, expressed as the percentage of positively stained cells (Labeling Percentage, LP), revealed highly significant differences among the experimental groups (one-way ANOVA: F(5,30) = 17.23; p < 0.001). The simultaneous combination therapy group (I4) exhibited the highest mean value (30.76 ± 1.34%), followed in descending order by tamoxifen monotherapy (I2: 29.35 ± 0.77%), sequential combination (I3: 28.37 ± 2.07%), Eleutherine bulbosa extract monotherapy (I1: 27.66 ± 0.90%), and the positive control (KP: 27.35 ± 0.98%). Post-hoc analysis using Tukey’s HSD test demonstrated that the I4 group was significantly superior to the KP, I1, and I3 groups (p < 0.05). In contrast, no significant difference was observed between the simultaneous combination (I4) and tamoxifen monotherapy (I2) groups (p > 0.05) (Table 3).
| Group | n | p53-positive cells (%) Mean ± SD | Tukey HSD (p < 0.05) * |
| Negative control (healthy, KN) | 6 | 34.10 ± 2.23 | a |
| Simultaneous combination (I4) | 6 | 30.76 ± 1.34 | b |
| Tamoxifen monotherapy (I2) | 6 | 29.35 ± 0.77 | b,c |
| Sequential combination (I3) | 6 | 28.37 ± 2.07 | c |
| Eleutherine bulbosa monotherapy (I1) | 6 | 27.66 ± 0.90 | c,d |
| Positive control (DMBA only, KP) | 6 | 27.35 ± 0.98 | d |
Footnote: • Data are expressed as mean percentage of p53-positive cells ± SD (n = 6 animals/group; 5 high-power fields per animal, total 30 observations). • Means with different superscript letters in the same column are significantly different (one-way ANOVA followed by Tukey’s HSD post-hoc test, F(5,30) = 17.23, p < 0.001). Means sharing the same letter do not differ significantly (p > 0.05)
Representative immunohistochemical micrographs of p53 expression in mammary tissue are shown in Figure 1. Stronger nuclear staining was observed in the simultaneous combination (I4) and tamoxifen monotherapy (I2) groups, in agreement with the quantitative results presented in Table 3.
Effect of ethanol of eleutherine bulbosa on morphology of breast tissue
Histopathological evaluation of mammary gland tissue revealed pronounced differences in alveolar morphology across treatment groups. The simultaneous combination therapy (I4) and tamoxifen monotherapy (I2) exhibited the largest mean alveolar diameters (106.71 µm and 101.29 µm, respectively) together with the lowest alveolar counts per field, closely approximating the values observed in healthy controls (110.40 µm and 1.6 alveoli/field). Conversely, the positive control group (DMBA-only, KP) displayed the smallest alveolar diameter (83.01 µm) and the highest alveolar density (2.6 alveoli/field), consistent with DMBA-induced hyperplasia (Table 4) shown in Figure 2.
| Sample Group | Mean Alveolar Diameter (µm) | Mean Alveolar |
| Healthy Control (KN) | 110.4 | 1.6 |
| Positive Control (KP) | 83.01 | 2.6 |
| I1 (Eleutherine bulbosa) | 86.25 | 2.4 |
| I2 (Tamoxifen) | 101.29 | 1.8 |
| I3 (Sequential) | 98.5 | 2 |
| I4 (Simultaneous) | 106.71 | 1.8 |
Footnote: • Histomorphometric data were derived from the average of five high-power fields (HPF, 400×) from a **single representative mammary tissue sample (n=1) per group**. Due to the single sample design, data are presented **descriptively** and were not subjected to inferential statistical analysis.
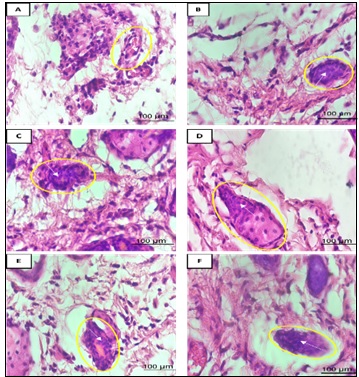
Figure 2. Histopathological Images of DMBA-induced Mammary Tissue in BALB/c Mice (H and E, 400×; scale bar = 100 µm). Yellow circles: mitotic cells; white arrows: degenerative/proliferative nuclei. Panels: (A) Normal Control (KN): intact alveoli; (B) Positive Control (KP): severe disruption/tumor infiltration; (C) Eleutherine bulbosa monotherapy (I1): partial recovery; (D) Tamoxifen monotherapy (I2): moderate improvement;(E) Sequential combination (I3): intermediate restoration;(F) Simultaneous combination (I4): greatest recovery,near-normal morphology .
Discussion
Therapeutic efficacy in the DMBA model can be reflected by improvements in tissue architecture. In this study, mean alveolar diameter and alveolar grade effectively indicated a shift from hyperproliferative changes toward tissue recovery. The simultaneous combination group (I4) showed the greatest histological improvement, despite having p53 levels comparable to tamoxifen monotherapy.
These findings suggest that the extract exerts additional tissue-level effects beyond p53 modulation, which are further explored in the following discussion.
Phytochemical characterization of extracts
Phytochemical characterization confirmed that the ethanolic extract of Eleutherine bulbosa contained substantial amounts of phenolic compounds and flavonoids, with total polyphenol and flavonoid contents of 1.5231% (GAE) and 0.9188% (QE), respectively. Both classes of compounds have been widely reported to exhibit antioxidant and antiproliferative effects in various cancer models, including breast cancer [23].
Polyphenols and flavonoids derived from natural sources are widely known to influence p53 stability and activity. Mechanistic studies suggest these compounds may support p53 function, possibly by inhibiting MDM2-mediated proteasomal degradation of p53 or by inducing mild oxidative stress within tumor cells [24]. This mild stress, in turn, can trigger DNA-damage responses that indirectly activate the p53 pathway [25, 26]. The clear increase in nuclear p53 immunoreactivity observed across the treatment groups, particularly in the simultaneous combination (I4) and tamoxifen monotherapy (I2) groups, is consistent with the known mechanisms of these bioactive molecules.
These findings align with the observations of Milliana et al. (2023), who previously demonstrated that Eleutherine bulbosa extract modulates the p53 pathway in breast cancer cells [27]. The improved histopathological features and stronger p53 nuclear staining seen in the simultaneous combination and tamoxifen-only groups may reflect a supportive role of the extract’s phenolic constituents in enhancing tumor-suppressor activity, although further molecular studies are required to confirm the exact contribution [28]. Effect of the ethanolic extract of Eleutherine bulbosa on p53 protein levels Comprehensive analysis of p53 in this DMBA-induced breast cancer mouse model revealed a consistent pattern in both systemic and tissue-level measurements. The positive control group (KP, DMBA only) exhibited the lowest plasma p53 concentration (3.62 ± 0.20 pg/mL) and the weakest nuclear p53 immunoreactivity (27.35 ± 0.98%), reflecting the well-documented functional inactivation of p53 under oncogenic stress induced by DMBA [21, 29]. All treatment groups showed significantly higher p53 levels than the positive control (p < 0.001). However, no statistically significant difference was observed between tamoxifen monotherapy (I2) and the simultaneous combination of tamoxifen plus Eleutherine bulbosa extract (I4) in either plasma p53 (p = 0.069) or the percentage of p53-positive cells on immunohistochemistry (I2: 29.35 ± 0.77%; I4: 30.76 ± 1.34%; p > 0.05). This indicates that adding the Eleutherine bulbosa extract to the simultaneous regimen did not produce a further measurable increase in overall p53 expression or stability. Despite the comparable p53 profiles, the simultaneous combination group (I4) consistently displayed the highest degree of morphological restoration compared with tamoxifen alone larger alveolar diameters, lower alveolar density, and reduced hyperplastic and necrotic changes. These findings suggest that the additional benefit provided by the extract is largely mediated through local actions within the mammary tissue rather than by a global enhancement of p53 levels [30]. The phenolic and flavonoid constituents of the extract are likely contributing via complementary mechanisms such as antioxidant, anti-inflammatory, or direct antiproliferative effects that are not fully captured by systemic or total nuclear p53 measurements [31]. The present results align with the observations of Milliana et al. (2023), who identified the p53 pathway as one of the targets modulated by Eleutherine bulbosa, while noting that other pathways are also involved. Taken together, the therapeutic advantage of the simultaneous combination appears to stem from multi-target, tissue-specific actions of the extract rather than from a simple additive effect on p53 itself [27, 32–34]. Effect of the ethanolic extract of Eleutherine bulbosa on mammary tissue morphology Histomorphometric evaluation of the mammary gland clearly showed that giving the Eleutherine bulbosa extract together with tamoxifen (I4) produced the best reversal of the neoplastic changes caused by DMBA. In the positive control animals (KP), alveolar diameter was sharply reduced (83.01µm) and alveolar density was high (2.6 alveoli/field), typical of uncontrolled hyperplasia. By comparison, healthy controls (KN) had spacious simultaneous combination and tamoxifen-only groups may reflect a supportive role of the extract’s phenolic constituents in enhancing tumor-suppressor activity, although further molecular studies are required to confirm the exact contribution [28].
Effect of the ethanolic extract of Eleutherine bulbosa on p53 protein levels
Comprehensive analysis of p53 in this DMBA-induced breast cancer mouse model revealed a consistent pattern in both systemic and tissue-level measurements. The positive control group (KP, DMBA only) exhibited the lowest plasma p53 concentration (3.62 ± 0.20 pg/mL) and the weakest nuclear p53 immunoreactivity (27.35 ± 0.98%), reflecting the well-documented functional inactivation of p53 under oncogenic stress induced by DMBA [21, 29]. All treatment groups showed significantly higher p53 levels than the positive control (p < 0.001). However, no statistically significant difference was observed between tamoxifen monotherapy (I2) and the simultaneous combination of tamoxifen plus Eleutherine bulbosa extract (I4) in either plasma p53 (p = 0.069) or the percentage of p53-positive cells on immunohistochemistry (I2: 29.35 ± 0.77%; I4: 30.76 ± 1.34%; p > 0.05). This indicates that adding the Eleutherine bulbosa extract to the simultaneous regimen did not produce a further measurable increase in overall p53 expression or stability.
Despite the comparable p53 profiles, the simultaneous combination group (I4) consistently displayed the highest degree of morphological restoration compared with tamoxifen alone larger alveolar diameters, lower alveolar density, and reduced hyperplastic and necrotic changes. These findings suggest that the additional benefit provided by the extract is largely mediated through local actions within the mammary tissue rather than by a global enhancement of p53 levels [30]. The phenolic and flavonoid constituents of the extract are likely contributing via complementary mechanisms such as antioxidant, anti-inflammatory, or direct antiproliferative effects that are not fully captured by systemic or total nuclear p53 measurements [31].
The present results align with the observations of Milliana et al. (2023), who identified the p53 pathway as one of the targets modulated by Eleutherine bulbosa, while noting that other pathways are also involved. Taken together, the therapeutic advantage of the simultaneous combination appears to stem from multi-target, tissue-specific actions of the extract rather than from a simple additive effect on p53 itself [27, 32–34].
Effect of the ethanolic extract of Eleutherine bulbosa on mammary tissue morphology
Histomorphometric evaluation of the mammary gland clearly showed that giving the Eleutherine bulbosa extract together with tamoxifen (I4) produced the best reversal of the neoplastic changes caused by DMBA. In the positive control animals (KP), alveolar diameter was sharply reduced (83.01µm) and alveolar density was high (2.6 alveoli/field), typical of uncontrolled hyperplasia. By comparison, healthy controls (KN) had spacious, well-formed alveoli with a mean diameter of 110.40 µm and only 1.6 alveoli per field.
When the extract was given alone (I1), the improvement was modest (alveolar diameter 86.2 µm), confirming that monotherapy is not enough to overcome severe carcinogenic damage. Tamoxifen alone (I2) performed much better, restoring alveolar diameter to 101.29 µm and bringing the alveolar count down to 1.8 per field, results that fit with its known ability to block estrogen- driven growth [35, 36]. The simultaneous combination (I4), however, came closest to normal tissue, with an average alveolar diameter of 106.71 µm and the same alveolar count as tamoxifen alone. The micrographs (Figure 2F) clearly illustrate the results: the I4 group’s alveoli exhibited a well-shaped shape, mitoses were infrequent, and necrosis was nearly non-existent a marked improvement over the results observed with tamoxifen alone (Figure 2D).
The sequential regimen (I3) lagged behind (98.50 µm), underlining how important timing is: giving the two agents together clearly creates a better environment for tissue recovery than giving them one after the other.
Interestingly, plasma p53 levels and nuclear p53 staining were statistically similar between tamoxifen alone (I2) and the simultaneous combination (I4). The superior histology in I4 therefore points to local actions of the extract inside the mammary tissue rather than any further boost in p53 expression. The phenolic and flavonoid compounds in the extract are well known for their antioxidant, anti-inflammatory, and direct antiproliferative effects at the tissue level, and these properties probably account for the extra architectural improvement we observed [37, 38].
Our findings agree with earlier work on Eleutherine bulbosa in the same DMBA model, where the extract lowered tumor burden and restored tissue structure without relying on any single pathway (Efrem et al., 2024; Milliana et al., 2023). Taken together, the results suggest that a standardized ethanolic extract of Eleutherine bulbosa, when administered concurrently with tamoxifen, can serve as a useful adjuvant, helping to improve therapeutic outcomes at the tissue level and bringing mammary gland morphology remarkably close to normal [22].
Clinical implications: Suggest the potential value of Eleutherine bulbosa as a promising standardized phytopharmaceutical adjuvant to enhance the tamoxifen response in ER⁺ breast cancer, particularly in early resistance settings. The voucher specimen (MUL- EB-2024) and ethical compliance (No. 071/UN4.14.1/ TP.01.02/2025) support reproducibility and translational potential.
Study Limitations
• Single-dose regimens restrict dose-response interpretation.
• Mechanistic pathways, including p53-associated signaling, were not directly measured.
• The use of one representative sample for histopathology limits inferential statistics.
Future Directions
• Molecular pathway profiling and apoptosis marker analysis.
• Validation in orthotopic or PDX breast cancer models.
• Dose optimization and pharmacokinetic evaluation of Eleutherine bulbosa.
Research Implications
This study contributes preliminary evidence supporting integrative therapeutic approaches for ER⁺ breast cancer. With a registered voucher specimen (MUL-EB-2024) and standardized extract (0.9188% QE flavonoids, 1.5231% GAE phenolics), provide groundwork for further preclinical validation. The integration of Eleutherine bulbosa with tamoxifen offers a polypharmacological model that may reduce chemotherapy doses, suppress early resistance, and enhance patient adherence through culturally rooted ethnomedicine (Dayak tradition).
In conclusion, simultaneous administration of Eleutherine bulbosa extract with tamoxifen yielded enhanced mammary tissue recovery in a DMBA-induced model, despite similar systemic p53 responses to tamoxifen alone. This suggests complementary, localized therapeutic effects that support further investigation into its potential as an adjuvant in breast cancer management.
Funding Sources
This research has not received financial support from any sponsor or funding institution.
Ethical Approval
This study was approved by an independent animal research ethics committee and was conducted in accordance with the guidelines of the Institutional Animal Care and Use Committee (IACUC) for the care and use of experimental animals (Ethical Clearance No. 071/ UN4.14.1/TP.01.02/2025).
Author’s contribution
Conceptualization: Fifin Ernawati. Methodology: Fifin Ernawati, Andi Ariyandy. Validation: Andi Nilawati Usman. Formal Analysis: Risfah Yulianty. Investigation: Fifin Ernawati. Data Curation: Andi Ariyandy. Writing— Original Draft: Fifin Ernawati. Writing—Review & Editing: Andi Nilawati Usman, Andi Ariyandy. Visualization: Risfah Yulianty
Data Availability
The research data are not publicly available due to ethical considerations regarding the use of experimental animals. Data may be provided by the corresponding author upon reasonable request.
AI Generative Tool Declaration
Generative artificial intelligence was not used in the preparation of this manuscript. All analyses, interpretations, and writing were performed solely by the author based on the conducted research.
Originality Declaration for Figures
All figures included in this manuscript are original and have been created by the authors specifically for the purposes of this study. No previously published or copyrighted images have been used. The authors confirm that all graphical elements, illustrations, and visual materials were generated from the data obtained in the course of this research or designed uniquely for this manuscript.
References
- The anticancer mechanism of action of selected polyphenols in triple-negative breast cancer (TNBC) Farghadani R, Naidu R. Biomedicine & Pharmacotherapy.2023;165. CrossRef
- Recent advancement in developing small molecular inhibitors targeting key kinase pathways against triple-negative breast cancer Islam R, Yen KP , Rani NNIM , Hossain MS . Bioorganic & Medicinal Chemistry.2024;112. CrossRef
- Targeting p53 pathways: mechanisms, structures and advances in therapy Wang H, Guo M, Wei H, Chen Y. Signal Transduction and Targeted Therapy.2023;8(1). CrossRef
- RNA-binding proteins in breast cancer: Biological implications and therapeutic opportunities Wang S, Sun H, Chen G, Wu C, Sun B, Lin J, Lin D, et al . Critical Reviews in Oncology/Hematology.2024;195. CrossRef
- Breast Cancer Treatments: Updates and New Challenges Burguin A, Diorio C, Durocher F. Journal of Personalized Medicine.2021;11(8). CrossRef
- Promoting Apoptosis, a Promising Way to Treat Breast Cancer With Natural Products: A Comprehensive Review Yuan L, Cai Y, Zhang L, Liu S, Li P, Li X. Frontiers in Pharmacology.2022;12. CrossRef
- Cancer in Southeast Asia: A comparative analysis of 2022 incidence and mortality data. Dee ECC , Eala MA , Laversanne M, Ginsburg O, Gomez SL , Feliciano EJG , Ng K, et al . Journal of Clinical Oncology.2024;42(16_suppl). CrossRef
- Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL , Soerjomataram I, Jemal A. CA: A Cancer Journal for Clinicians.2024;74(3). CrossRef
- Therapeutic effect of simvastatin on DMBA‐induced breast cancer in mice Karimi B, Ashrafi M, Shomali T, Yektaseresht A. Fundamental & Clinical Pharmacology.2019;33(1). CrossRef
- Breast cancer Harbeck N, Penault-Llorca F, Cortes J, Gnant M, Houssami N, Poortmans P, Ruddy K, Tsang J, Cardoso F. Nature Reviews Disease Primers.2019;5(1). CrossRef
- TNFα Enhances Tamoxifen Sensitivity through Dissociation of ERα-p53-NCOR1 Complexes in ERα-Positive Breast Cancer Kim H, Park S, Lee J, Sung G, Song J, Kwak S, Jeong J, et al . Cancers.2021;13(11). CrossRef
- Identification of novel functional compounds from forest onion and its biological activities against breast cancer Nurkolis F, Kurniatanty I, Wiyarta E, Permatasari HK , Mayulu N, Taslim NA , Tjandrawinata RR , et al . Journal of Agriculture and Food Research.2024;18. CrossRef
- New naphtoquinones derivatives from the edible bulbs of Eleutherine americana and their protective effect on the injury of human umbilical vein endothelial cells Chen D, Qiao J, Sun Z, Liu Y, Sun Z, Zhu N, Xu X, Yang J, Ma G. Fitoterapia.2019;132. CrossRef
- Alpha-Glucosidase Inhibitory Activity and Analysis of Eleuthoside B from Eleutherine bulbosa Urb. Bulbs Purified Extract from Lampo Donggala, Central Sulawesi, Indonesia Anam S, Yuyun Y, Tanaijo S, Langkoda F, Yusriadi Sultan A, et al . Tropical Journal of Natural Product Research.2025;7(9). CrossRef
- Eleutherine bulbosa (Mill.) Urb. Bulb: Review of the Pharmacological Activities and Its Prospects for Application Kamarudin AA , Sayuti NH , Saad N, Razak NAA , Esa NM . International Journal of Molecular Sciences.2021;22(13). CrossRef
- Induction of apoptosis by Eleutherine bulbosa (Mill.) Urb. bulb extracted under optimised extraction condition on human retinoblastoma cancer cells (WERI-Rb-1) Kamarudin AA , Sayuti NH , Saad N, Razak NAA , Esa NM . Journal of Ethnopharmacology.2022;284. CrossRef
- Total flavonoid content revised: An overview of past, present, and future determinations in phytochemical analysis Nicolescu A, Bunea CI , Mocan A. Analytical Biochemistry.2025;700. CrossRef
- Implementation of an Analytical Method for Spectrophotometric Evaluation of Total Phenolic Content in Essential Oils Michiu D, Socaciu M, Fogarasi M, Jimborean AM , Ranga F, Mureşan V, Semeniuc CA . Molecules.2022;27(4). CrossRef
- A multicentre study on spontaneous in-cage activity and micro-environmental conditions of IVC housed C57BL/6J mice during consecutive cycles of bi-weekly cage-change Ulfhake B., Lerat H., Honetschlager J., Pernold K., Rynekrová M., Escot K., Recordati C., et al . PLOS ONE.2022;17(5). CrossRef
- DMBA-induced Modulate Estrogen Receptors α and β Breast Cancer’s Animal Model Rosdianto AM , Kurniawan A, Gunadi JW , Mahendra I, Setiawan I, Goenawan H, Sylviana N, et al . Majalah Kedokteran Bandung.2022;54(1). CrossRef
- Salvadora persica attenuates DMBA-induced mammary cancer through downregulation oxidative stress, estrogen receptor expression and proliferation and augmenting apoptosis Hamza AA , Khasawneh MA , Elwy HM , Hassanin SO , Elhabal SF , Fawzi NM . Biomedicine & Pharmacotherapy.2022;147. CrossRef
- Chemopreventive effect of dayak onion [Eleutherine bulbosa, Mill. (Urb)] against 7,12-dimethylbenz [α] anthracene (DMBA)-induced breast cancer in rats: study on cancer antigen 15-3 (CA 15-3) Efrem DG , Ilmiawan MI , Pratiwi SE . Indonesian Journal of Biomedicine and Clinical Sciences.2024;56(3). CrossRef
- The Therapeutic Potential of Laurus nobilis L. Leaves Ethanolic Extract in Cancer Therapy Al-Mammori F, Qasem AMA , Al-Tawalbeh D, Abuarqoub D, Hmedat A. Molecules.2025;30(19). CrossRef
- The effectiveness of exercise on the symptoms in breast cancer patients undergoing adjuvant treatment: an umbrella review of systematic reviews and meta-analyses Zhao Y, Tang L, Shao J, Chen D, Jiang Y, Tang P, Wang X. Frontiers in Oncology.2023;13. CrossRef
- P53 Expression in benign Breast Disease Development: A Systematic Review Senra RLS , Silva IFD . Asian Pacific Journal of Cancer Prevention.2020;21(9). CrossRef
- Effectiveness of internet-based support interventions on patients with breast cancer: a systematic review and narrative synthesis Huang Y, Li Q, Zhou F, Song J. BMJ Open.2022;12(5). CrossRef
- The Potential of Eleutherine bulbosa in Inducing Apoptosis and Inhibiting Cell Cycle in Breast Cancer: A Network Pharmacology Approach and In Vitro Experiments Milliana A, Listiyana A, Mutiah R, Annisa R, Firadusi A, Faradila V, Febriani A, et al . Asian Pacific Journal of Cancer Prevention.2023;24(11). CrossRef
- Discrepancies in breast cancer guideline recommendations despite similar Cochrane systematic review conclusions Zhang Z, Cheng J, Hou J, Niu M, Gao Y, Xu J, Zheng Q, et al . Journal of Evidence-Based Medicine.2024;17(1). CrossRef
- Protective Effects of Pelargonidin against DMBA-Induced Mammary Tumorigenesis in BALB/c Mice through Reduced Oxidative Stress and Lipid Anomalies Laskar YB , Bhattacharjee K, Nath M, Choudhury Y, Mazumder PB , Talukdar AD . Nutrition and Cancer.2023;75(7). CrossRef
- Potential Protective Effect of Achillea fragrantissima against Adriamycin-Induced Cardiotoxicity in Rats via an Antioxidant and Anti-Inflammatory Pathway Hijazi MA , Jambi HA , Aljehany BM , Althaiban MA . BioMed Research International.2019;2019. CrossRef
- Phytochemical screening and antioxidant activity of Uncaria tomentosa extract: In vitro and in vivo studies Oubaid EN , Abu-Raghif AR , Al-Sudani IM . Medical Journal of Babylon.2023. CrossRef
- Recent Advances in the Treatment of Breast Cancer Tong CWS , Wu M, Cho WCS , To KKW . Frontiers in Oncology.2018;8. CrossRef
- Bio-vehicles of cytotoxic drugs for delivery to tumor specific targets for cancer precision therapy Al-mansoori L, Elsinga P, Goda SK . Biomedicine & Pharmacotherapy.2021;144. CrossRef
- Apoptosis Deregulation and the Development of Cancer Multi-Drug Resistance Neophytou CM , Trougakos IP , Erin N, Papageorgis P. Cancers.2021;13(17). CrossRef
- Impacting the Remedial Potential of Nano Delivery-Based Flavonoids for Breast Cancer Treatment Sindhu RK , Verma R, Salgotra T, Rahman MH , Shah M, Akter R, Murad W, et al . Molecules.2021;26(17). CrossRef
- Estrogenic flavonoids and their molecular mechanisms of action Kiyama R. The Journal of Nutritional Biochemistry.2023;114. CrossRef
- Honey and its nutritional and anti-inflammatory value Ranneh Y, Akim AM , Hamid HA , Khazaai H, Fadel A, Zakaria ZA , Albujja M, Bakar MFA . BMC Complementary Medicine and Therapies.2021;21(1). CrossRef
- Phytochemical analysis and antibacterial activities of Eleutherine bulbosa (Mill.) Urb. extract against Vibrio parahaemolyticus Munaeni W, Yuhana M, Setiawati M, Wahyudi AT . Asian Pacific Journal of Tropical Biomedicine.2019;9(9). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2026
Author Details