Prognostic Significance of MGMT Promoter Methylation in Egyptian GBM Patients: A Single-institution Experience
Download
Abstract
Background: Glioblastoma Multiforme (GBM) is the highest-mortality tumor of the central nervous system. Epigenetic silencing of the MGMT gene by promoter methylation is associated with loss of MGMT expression and deficiency in MGMT-mediated DNA repair, which is affiliated with improved survival in patients treated with alkylating agents such as TMZ.
Purpose: This is a retrospective work, studying the MGMT promoter status in a group of GBM patients; correlating this status to time to progression (TTP) and overall survival (OS).
Methods: Thirty-nine patients with GBM, treated in Kasr El-Ainy Center of Clinical Oncology and Nuclear Medicine (NEMROCK) between January 2014 and January 2015, were included in our study. The QIAamp DNA FFPE Tissue Kit (Qiagen, USA) was used for genomic DNA extraction from Formalin-fixed paraffin-embedded tumor tissues of the 39 patients. Bisulfite modification of DNA was performed for detecting methylation in MGMT promoter using EpiTect Bisulfite Kit (Qiagen, USA). Specific primers were used to match methylated & un-methylated DNA that was visualized by loading its PCR products in gel electrophoresis system. All statistical analyses were carried out using SPSS version 20.0.
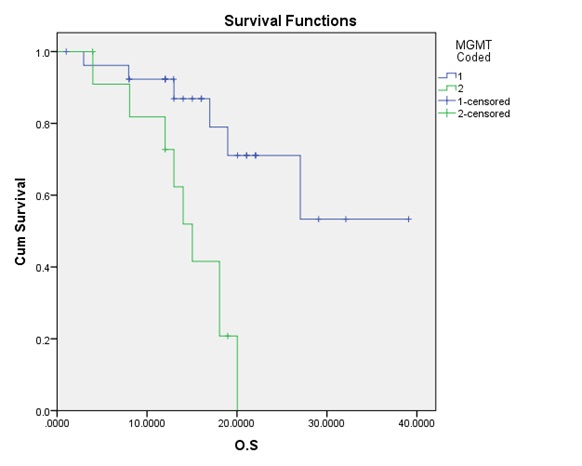
Results: The mean age for our patients was 48 years; with a male to female ratio of 1.3:1. MGMT promoter methylation was found in 27 patients (69.2%) compared to 12 (30.8%) with un-methylation. TMZ was received in 71.8 % (28) of our patients throughout their treatment. The median OS for all patients was 20.03 months; while the median TTP 15.03 was months. Although the OS was statistically significantly higher for patients with the methylated promoter (p-value = 0.004) compared to the un-methylated group; yet the TTP difference did not reach a statistically significant value (p-value = 0.048).
Conclusions: The epigenetic silencing of the MGMT gene by promoter methylation has been associated with longer OS & TTP in patients with GBM.
Introduction
Glioblastoma Multiforme (GBM) is the highest-mortality tumor of the central nervous system. The role of temozolomide (TMZ), along with maximal safe resection and radiation, was established for the treatment of newly diagnosed GBM patients years ago [1].
The MGMT (O6-methylguanine–DNA methyltransferase) gene is located on chromosome 10q26 and encodes a protein which is responsible for DNA repair via removal of the alkyl groups from the O 6 position of guanine, an important site of DNA alkylation. The unrepaired, chemotherapy-induced lesions trigger cytotoxicity and apoptosis [2]. The high activity level of MGMT in the malignant cells creates resistance by blunting the therapeutic effect of alkylating agents and may be an important determinant of treatment failure [3]. Epigenetic silencing of the MGMT gene by promoter methylation is associated with loss of MGMT expression and result in a deficiency in MGMT-mediated DNA repair [4].
Subset analyses had confirmed the sensitivity of tumors deficient in MGMT (defined by MGMT promoter methylation) to temozolomide, compared to those with adequate MGMT expression (defined by an un-methylation promoter) [5]. Several reports have concluded that epigenetic silencing is affiliated with improved survival in GBM patients who were treated with alkylating agents such as TMZ [6].
This is a retrospective work, studying the MGMT promoter status in a group of GBM patients; correlating this status to time to progression (TTP) and overall survival (OS).
Materials and Methods
Thirty-nine patients with GBM, who had been treated in Kasr El-Ainy Center of Clinical Oncology and Nuclear Medicine (NEMROCK) in the period between January 2014 and January 2015, were included in our study. All the patients (39) underwent surgical intervention followed by postoperative radiation therapy. Temozolomide (TMZ) was used only in 28 patients as concurrent and adjuvant treatment.
MGMT testing
The Formalin-fixed paraffin-embedded tumor tissue blocks were collected from the patients. The QIAamp DNA FFPE Tissue Kit (Qiagen, USA) was used for genomic DNA extraction from Formalin-fixed paraffin-embedded tumor tissues of the 39 patients. Bisulfite modification of DNA was performed for detecting methylation in MGMT promoter using EpiTect Bisulfite Kit (Qiagen, USA). Specific primers were used to match methylated & un-methylated DNA that was visualized by loading its PCR products in gel electrophoresis system.
Statistical analysis
Data analyses were carried out using statistical package for social sciences (SPSS), program version 20. All data entries were checked for accuracy against the original raw data of each patient. The significance level of all statistical analysis was at < 0.005 (P-value).
Results
Patients’ and tumor’s characteristics were collected and recorded as shown in Tabl 1. The mean age for our patients was 48 years; with a male to female ratio of 1.3:1. MGMT promoter methylation was found in 27 patients (69.2%) compared to 12 (30.8%) with un-methylation. All patients (39) received postoperative radiation therapy. TMZ was received in 71.8 % (28) of our patients throughout their treatment.
| Number | Percent | |
| Age (years): < 48 ≥ 48 Median (range) | 16 22 48 | 41% 59% |
| Sex: Male Female | 22 17 | 56.4% 43.6% |
| Site of lesion: Frontal Fronto-parietal Parietal Temporal Tempo-parietal Occipital | 4 12 1 1 15 6 | 10.3% 30.8% 2.6% 2.6% 38.5% 15.4% |
| Type of Surgery: Total resection Debulking Surgery Biopsy | 6 26 8 | 15.4% 64.1% 20.5% |
| MGMT Status: Methylated Un-methylated | 27 12 | 69.2% 30.8% |
| Variable | OS (months) |
| Median | 20.03 |
| 95%CI N of Event | (11.61-28.45) 15 |
| 6ms Survival rate | 89.75% |
Figure 1 :The overall survival (OS) curve
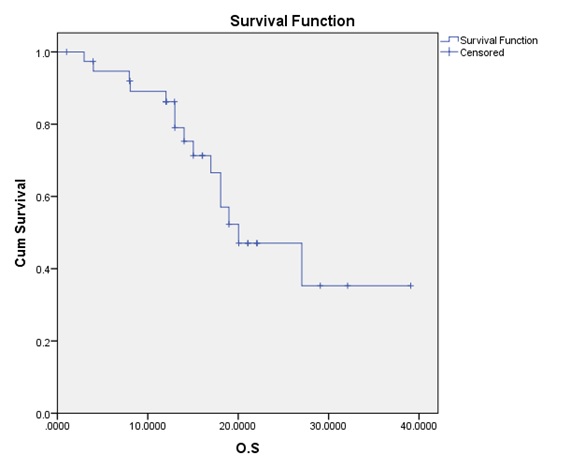
| Variable | T.T.P (months) |
| Median | 15.03 |
| 95%CI N of Event | (08.91-21.15) 23 |
| 6ms Survival rate | 71.79% |
Figure 2 :The time to progression (TTP) survival curve.
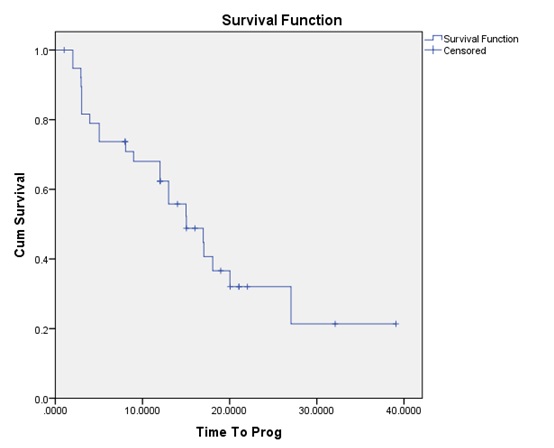
The median OS for all patients was 20.03. months; while the median TTP 15.03 was months. Although the OS was statistically significantly higher for patients with the methylated promoter (p-value = 0.004) compared to the un-methylated group; yet the TTP difference did not reach a statistically significant value (p-value = 0.048).
Figure 3 :The correlation between the OS and the MGMT promoter methylation.
Discussion
Alkylating agents, including temozolomide and the nitrosoureas (carmustine and lomustine), are commonly used cytotoxic chemotherapies for newly diagnosed as well as recurrent GBM patients. They cause apoptosis by methylating guanine at the O6 position, initiating a double-strand break in the DNA, and cell death [7]. The MGMT protein repairs the DNA damage via removing the damaged alkyl groups from the O6 position of guanine. The alkylated protein is then degraded, requiring constant refillment for DNA repair to be fruitful [8]. High expression of MGMT in cancer cells accounts for the primary resistance against alkylating agents [9,10].
Setting the cutoff between MGMT methylation and unmethylation, as defined by methylation-specific PCR, is a little bit complicated by methylation pattern, heterogeneity, and tissue contamination with non-neoplastic-methylated cells. This leads to wide variability among laboratories and lack of standardization, which makes the interpretation of the prognostic and predictive impact of MGMT methylation status difficult. However, the constancy of results showing improved outcomes for methylated patients, irrespective to the treatment given, and an adjoined benefit with temozolomide, suggest that MGMT methylation has both prognostic and predictive impact [11].
The NOA-08 trial was a large, phase 3 study planned to experiment temozolomide monotherapy in patients with newly diagnosed high-grade gliomas >65 years of age. The design was to compare it as a non-inferior treatment to standard radiation (60 Gy). They tested 51 % of samples for MGMT promoter methylation using two distinct methylation-specific PCRs. The authors found that MGMT promoter was methylated in 35% of tested samples; methylation status as a predictive marker of response to temozolomide. They suggested temozolomide monotherapy in elderly patients who are MGMT methylation versus radiation therapy alone in those unmethylated [12].
In the Nordic trial, temozolomide’s efficacy was tested as a monotherapy to standard radiation (60 Gy) and hypofractionated radiation (34 Gy in 3– 4 Gy over 2 weeks) in newly diagnosed GBM patients >60 years of age. MGMT methylation status was evaluated in 59 % of patients, and 45 % of the samples were found to be methylated [13]. Results supported previous evidence demonstrating the value of hypofractionated radiation (40 Gy) in elderly patients [14]. They reached similar conclusions to those from the NOA-08 study, with inferior mOS in patients with unmethylated MGMT, regardless of treatment, and no gained survival benefit with chemotherapy [13].
Another study was performed to interpret the prognostic and predictive value of MGMT promoter methylation in Chinese patients with GBM. It is considered a powerful study because of the large sample recruited, its prospective design, the standardization use, and pyrosequencing analysis to assess the MGMT promoter methylation status, and the chance of dissecting the prognostic and predictive aspects of MGMT promoter methylation as a biomarker. This cohort represents patients treated in a single center over a 68-month period [15]. The group with methylation data had a similar median age and range, performance status, and percent of patients with biopsy vs debulking surgery compared with other clinical studies [16–19]. Progression-free survival was 8.2 months compared with 6.9 months reported by SteelFisher et al; as for the overall survival, it was 13.1 months in contrast to 14.6 months. MGMT promoter methylation was prognostic for OS but not for PFS for the whole group of 128 participants [17].in Conclusion,The epigenetic silencing of the MGMT gene by promoter methylation has been associated with longer OS & TTP in patients with GBM.
Acknowledgments
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. The authors declare no conflict of interest.
References
[1]. Fabi A, Russillo M, Metro G, Vidiri A, Di Giovanni S, Cognetti F. Pseudoprogression and MGMT status in glioblastoma patients: implications in clinical practice. Anticancer Res. 2009;29(7):2607-10.
[2]. Ochs K, Kaina B. Apoptosis induced by DNA damage O6-methylguanine is Bcl-2 and caspase-9/3 regulated and Fas/caspase-8, independent. Cancer Res. 2000;60(20):5815- 24.
[3]. Gerson SL. MGMT: its role in cancer aetiology and cancer therapeutics. Nat Rev Cancer. 2004;4(4):296-307.
[4]. Esteller M, Hamilton SR, Burger PC, Baylin SB, Herman JG. Inactivation of the DNA repair gene O6-methylguanine-DNA methyltransferase by promoter hypermethylation is a common event in primary human neoplasia. Cancer Res. 1999;59(4):793-7.
[5]. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
[6]. Motomura K, Natsume A, Kishida Y, Higashi H, Kondo Y, et al. Benefits of interferon-b and temozolomide combination therapy for newly diagnosed primary glioblastoma with the unmethylated MGMT promoter: A multicenter study. Cancer 2011; 117(8): 1721–1730.
[7]. Liu L, Markowitz S, Gerson SL. Mismatch repair mutations override alkyltransferase in conferring resistance to temozolomide but not to 1,3-bis (2-chloroethyl) nitrosourea. Cancer Res. 1996;56 (23): 5375–9.
[8]. Pegg AE. Repair of O(6)-alkylguanine by alkyltransferase. Mutat Res. 2000;462 (2–3):83–100.
[9]. BelanichM, PastorM, Randall T, Guerra D, Kibitel J, Alas L, et al. Retrospective study of the correlation between the DNA repair protein alkyltransferase and survival of brain tumor patients treated with carmustine. Cancer Res. 1996;56 (4):783–8.
[10]. Jaeckle KA, Eyre HJ, Townsend JJ, Schulman S, Knudson HM, Belanich M, et al. Correlation of tumor O6 methylguanine-DNA methyltransferase levels with survival of malignant astrocytoma patients treated with bis-chloroethylnitrosourea: a Southwest Oncology Group study. J Clin Oncol. 1998;16(10):3310-5.
[11]. Wick W, Weller M, van den Bent M, Sanson M, Weiler M, von Deimling A, et al. MGMT testing--the challenges for biomarker-based glioma treatment. Nat Rev Neurol. 2014;10(7):372-85.
[12]. WickW, Platten M, Meisner C, Felsberg J, Tabatabai G, Simon M, et al. Temozolomide chemotherapy alone versus radiotherapy alone for malignant astrocytoma in the elderly: the NOA-08 randomized, phase 3 trial. Lancet Oncol. 2012;13 (7):707–15.
[13]. Malmstrom A, Gronberg BH, Marosi C, Stupp R, Frappaz D, Schultz H, et al. Temozolomide versus standard 6-week radiotherapy versus hypofractionated radiotherapy in patients older than 60 years with glioblastoma: the Nordic randomized, phase 3 trial. Lancet Oncol. 2012;13 (9):916–26.
[14].Roa W, Brasher PM, Bauman G, Anthes M, Bruera E, Chan A, et al. Abbreviated course of radiation therapy in older patients with glioblastoma multiforme: a prospective randomized clinical trial. J Clin Oncol. 2004;22(9):1583-8.
[15]. Dong Shen, Tao Liu, Qingfen Lin, et al. MGMT Promoter Methylation Correlates with an Overall Survival Benefit in Chinese High-Grade Glioblastoma Patients Treated with Radiotherapy and Alkylating Agent-Based Chemotherapy: A Single-Institution Study. PLOS ONE. September 2014; 9 (9), e107558.
[16]. Hegi ME, Diserens AC, Gorlia T, Hamou MF, de Tribolet N, Weller M, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997-1003.
[17]. SteelFisher GK, Blendon RJ, Bekheit MM, Mitchell EW, Williams J, et al. Novel pandemic A (H1N1) influenza vaccination among pregnant women: motivators and barriers. American Journal of Obstetrics and Gynecology. 2011; 204(6 Suppl 1):116–123.
[18]. Gerstner ER, Yip S, Wang DL, Louis DN, Iafrate AJ, et al. Mgmt methylation is a prognostic biomarker in elderly patients with newly diagnosed glioblastoma. Neurology. 2009; 73(18): 1509–1510.
[19. ]Kitange GJ, Carlson BL, Mladek AC, Decker PA, Schroeder MA, Wu W, et al. Evaluation of MGMT promoter methylation status and correlation with temozolomide response in orthotopic glioblastoma xenograft model. J Neurooncol. 2009;92(1):23-31.
License
Copyright
© ,
Author Details