Dominant and Recessive Genetic Models of LSP1 Gene rs3817198 Polymorphism and Breast Cancer Risk: A Systematic Review and Meta-analysis Study
Download
Abstract
Objective: The aim of this meta-analysis was to discover the effect of dominant and recessive genetic models of LSP1 gene rs3817198 polymorphism on breast cancer risk.
Material and Method: We performed this meta-analysis according to PRISMA protocol. Three databases, including PubMed/Medline, Web of sciences, and EMBASE were searched. In this meta-analysis, we included all studies that evaluated the association between LSP1 gene rs3817198 and breast cancer risk. ORs and their reported 95% confidence interval (CI) for dominant and recessive inheritance models were extracted from final retrieved studies. ORs were pooled using both fixed and the random-effect models. Egger’s test and contour-enhanced funnel plot were used to evaluate publication bias and small study effect. Twelve publications were eligible for final analysis after observing our defined inclusion and exclusion criteria.
Results: Totally, this meta-analysis composed of 15,530 cases and 20,258 controls. This study revealed a significant association between LSP1 gene rs3817198 polymorphism and breast cancer in the dominant genetic model (OR=1.07 [1.01-1.14]). Inversely, no association was found in the recessive genetic model (OR=1.10 [0.93-1.32]). Subgroup analysis displayed a significant association in population-based studies and European & American and African population only in the dominant genetic model. Begg’s funnel plot and Egger’s test revealed no publication bias.
Conclusion: According to our findings, it seems that LSP1 gene rs3817198 polymorphism is associated with breast cancer risk and this risk is more prominent in Caucasians.
Introduction
Breast cancer is the most common cancer among women and it is the second cause of cancer-related death. Environmental exposure along with genetic pre-disposition has been shown to have a cumulative effect on breast cancer risk [1]. Genetic component is responsible for 30-40% of familial and only 3-4% of the total number of breast cancer cases [2]. Recently, there has been a real effort to prove the role of single nucleotide polymorphisms (SNPs) in breast cancer risks [3, 4, 5].
Leukocyte-specific protein 1 (LSP1) gene is located on 11p15.5 and encodes an F-actin binding protein. LSP1 gene rs3817198T>C polymorphism has been studied in many kinds of literature. Several studies showed no association between rs3817198 SNP and breast cancer [6, 7, 8], but there are pieces of evidence on the relationship between rs3817198 SNP and increased [9, 10] or maybe decreased risk of breast cancer [11]. The aim of this meta-analysis was to predict the effect of dominant and recessive genetic models of LSP1 gene rs3817198 polymorphism on breast cancer risk.
Materials and Methods
Literature identification
We performed this meta-analysis according to PRISMA (Preferred Reporting Items for Systematic Reviews and Meta-Analyses) protocol. Three databases, including PubMed/Medline, Web of sciences, and EMBASE were searched not to miss any study. We employed phrases “LSP”, “LSP1”, “lymphocyte-specific protein”, “WP34” and “breast cancer”, “breast tumor”, and “breast neoplasm” for our search. Process of databases search is provided in supplementary Tabl 1. Reference list of final included studies and related meta-analyses were additionally searched for any possible missed citations.
| First author | Year | Menopausal | Dominant | Recessive | |||||
| status | |||||||||
| Adjusted OR | Unadjusted OR | Adjusted OR | Unadjusted OR | ||||||
| (95% CI) | (95% CI) | (95% CI) | (95% CI) | ||||||
| Barnholtz-Sloan, J. S. | 2010 | Mix | - | 1.07 (0.85-1.35)* | - | 0.43 (0.19-0.91)* | |||
| (African-American) | |||||||||
| Barnholtz-Sloan, J. S. | 2010 | Mix | - | 1.07 (0.91-1.27)* | - | 1.25 (0.95-1.66)* | |||
| (White) | |||||||||
| Gorodnova, T. V. | 2010 | Mix | 1.55 (0.97-2.49)* | 2.02 (0.93-4.46)* | |||||
| Latif, A. | 2010 | Mix | - | 0.90 (0.69-1.17)* | - | 1.04 (0.69-1.60)* | |||
| Tamimi, R. M. | 2010 | Mix | - | 1.10 (0.89-1.36)* | - | 1.13 (0.76-1.68)* | |||
| Campa, D. | 2011 | Mix | 1.16 (0.94-1.43) | - | 1.45 (0.81-2.57) | - | |||
| Sueta, A. | 2011 | Mix | - | 1.01 (0.91-1.11)* | - | 0.95 (0.89-1.00)* | |||
| Butt, S | 2012 | Mix | - | 1.16 (0.96-1.40)* | - | 1.47 (1.06-2.03)* | |||
| Shan, J. | 2012 | Mix | - | 1.20 (0.92-1.57)* | - | 1.30 (0.82-2.10)* | |||
| Mizoo, T. | 2013 | Mix | - | 1.18 (0.87-1.61)* | - | 1.97 (0.60-7.43)* | |||
| Pre | - | - | - | - | |||||
| Post | - | - | - | - | |||||
| Chen, Y. | 2016 | Mix | - | 0.41 (0.04-1.80)* | - | 0.58 (0.32-1.01)* | |||
| Deng, Z. | 2016 | Mix | 1.14 (0.73-1.77) | - | 3.80 (1.26-11.49) | - | |||
| Tan, T. | 2016 | Mix | - | 0.74 (0.20-2.35)* | - | 0.84 (0.63-1.13)* | |||
a) Year, year of publication; OR, odds ratio; CI, confidence interval; ⃰ ORs calculated by authors via STATA software
Study selection
We included all studies that evaluated the association between LSP1 gene rs3817198 and breast cancer risk in this meta-analysis. The exclusion criteria were: (1) evaluation of different polymorphisms of LSP1 gene; (2) no control group; (3) no measure of association or unavailable information for calculation odds ratio (OR); (4) breast cancer mortality and benign breast disease as main outcome; and (5) case report and animal studies.
Data extraction
Two authors (ASM, MR) independently screened citations and took all needed information. Name of first author, publication date, study design, source of controls (population-based or hospital-based), considered confounders in each model, genotyping methods, population ethnicity, total number of cases and controls, minor allele frequency and OR and their reported 95% confidence interval (CI) for dominant and recessive inheritance models were extracted from final retrieved studies.
Study qualification
In order to assess the quality of included studies, we considered four items including 1) source of the control group, 2) ethnicity, 3) menopausal status, and 4) sample size. Quality of studies based on mentioned four items is presented in supplementary Table 2. (Fig.6).
Figure 6 :(Table 2) Subgroup Meta-Analysis of Dominant and Recessive Genetic Model of LSP1 Rs3817198 Polymorphism and Breast Cancer Risk
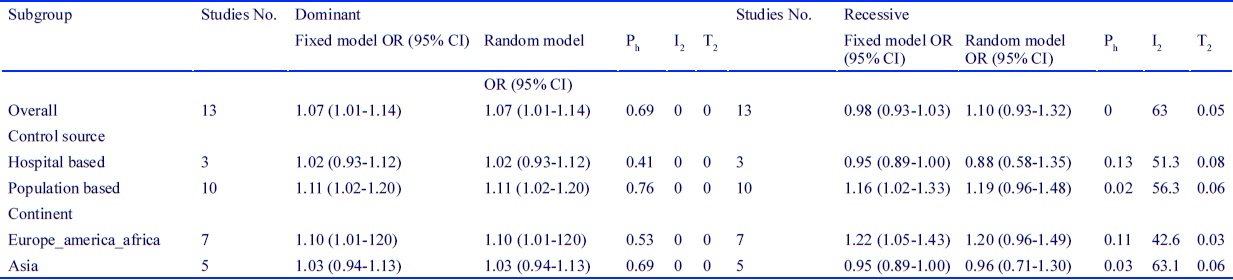
OR, odds ratio; CI, confidence intervals; Ph, P values of Q-test for heterogeneity test; I2, I-square; T2, Tau-square
Statistics
Minor Allele frequency for cases and controls were calculated for each study separately. The X2 statistic was used for Hardy–Weinberg equilibrium based on calculated frequencies of the LSP1 gene rs3817198 genotype. ORs were pooled using both fixed and the random-effect models. Heterogeneity across studies was evaluated by mean of Q and I2 statistics [12, 13]. An I2 value above 75% at a significance level of < 0.1 was considered as presence of statistically significant heterogeneity [14]. Egger’s test and contour-enhanced funnel plot were used to evaluate publication bias and small study effect [15]. The association of genetic inheritance models including dominant and recessive was assessed. Ethnicity and study setting were analyzed as subgroups. The ethnicity subgroup was defined based on the continent as Asians, Europeans, North Americans, and Africans. Subgroups were population-based (those reported a population-based case control or nested case-control) and hospital- based (those reported a hospital-based case-control). We employed Stata version 13 (Stata Corp LP, College Station, TX, USA) for all analyses.
Results
Selected literature
Our primary search yielded 287 citations, full-text of 26 publications were precisely assessed after evaluation and screening of citations. Twelve publications were eligible for final analysis after observing inclusion and exclusion criteria [7, 8, 10, 11, 16, 17, 18, 19, 20, 21, 22, 23]. Flowchart of the screening process is provided in (Fig. 1)
Figure 1 :Flowchart Diagram of Selection Process
Studies conducted by Barnholtz-Sloan (African-American and white) [11] was considered as two separate studies, because they provided ORs for two separate populations. Nine of studies had a population-based source of control. Four studies were conducted in Europe, one in North America, five in Asia, and one in Africa. Totally, this meta-analysis composed of 15,530 cases and 20,258 controls. Detailed characteristics of included and excluded studies are provided in supplementary Table 3-4.
Analysis
The main analysis of this study revealed a significant association between LSP1 gene rs3817198 polymorphism and breast cancer in the dominant genetic model (OR=1.07[1.01-1.14], (Fig. 2). Inversely, no association was found in the recessive genetic model (OR=1.10) [0.93-1.32], (Fig. 3). Additional subgroup analysis using the source of controls and ethnicity displayed a significant association in population-based studies and European and American and African population only in the dominant genetic model. However, the results on the recessive model indicated no relationship between breast cancer and LSP1 gene rs3817198 polymorphism. Table 2 presents complete outcome of this meta-analysis.
Figure 2 :Random Effect Model Meta-Analysis of LSP1 Gene rs3817198 Polymorphism for Dominant Genetic Models and Risk of Breast Cancer

Figure 3 :Random Effect Model Meta-Analysis of LSP1 Gene rs3817198 Polymorphism for Recessive Genetic Models and Risk of Breast Cancer. The Effect Size is Odds Ratio.
Publication bias
Begg’s funnel plot and Egger’s test were used for the assessment of publication bias, confirming no publication bias (Fig 4-5).
Figure 4 :Funnel Plot for Dominant Genetic Model with Regression Line Corresponding to the Egger’s Regression Test for Funnel-Plot Asymmetry
Figure 5 :Funnel Plot for Recessive Genetic Model with Regression Line Corresponding to the Egger’s Regression Test for Funnel-Plot Asymmetry
Discussion
Cancer is one of the leading causes of morbidity and mortality. During years, SNPs are always accused of synergic effects on cancer incidence. Many attempts have been made to clarify the role of SNPs in breast cancer risk [4, 11, 24] and it is still a topic of debate. LSP1 gene is contributing in the production of an F-actin binding protein, which is expressed lymphocytes, neutrophils, and endothelial cells. LSP1 gene rs3817198 polymorphism is one of the SNPs that have been studied in many kinds of literature and different results have come out [11, 16, 19]. This paper tried to integrate all available data and make it clear to readers that whether there is an association between LSP1 gene rs3817198 polymorphism and breast cancer or not.
We found a significant association between LSP1 gene rs3817198 polymorphism and breast cancer in the overall analysis, population-based studies, and European and American and African population only in the dominant genetic model. Results on analysis of recessive genetic model showed no relationship in overall and subgroup analysis.
Totally, two previous meta-analyses reported no association between LSP1 gene rs3817198 polymorphism and breast cancer [25, 26]. This was while our analysis yielded significant association for the dominant genetic model. In subgroup analysis, a study conducted by Chen et al [25] indicated this association only in Caucasian for the recessive genetic model. Another study by Tang et al [26] revealed a significant association among Caucasian in both recessive and dominant genetic model. In our study, it was revealed that LSP1 gene rs3817198 polymorphism increased breast cancer risk in European and American and African population (Caucasian) for the dominant genetic model. Besides, the latter demonstrated no association in population -based or hospital-based studies, but this paper showed that in population-based studies LSP1 gene rs3817198 polymorphism is associated with increased breast cancer risk. These all is maybe because we included more studies.
This study had some limitations that may cause our results to be interfered cautiously. First, adjusted and unadjusted ORs were analyzed together. Second, we were not able to perform subgroup analysis for other risk factors of breast cancer, including menopausal status, dietary intake, obesity, and smoking. Third, data about per allele ORs was not collected in this study and per allele OR was not calculated.
Finally, it seems that LSP1 gene rs3817198 polymorphism is associated with breast cancer risk and this risk is more prominent in Caucasians. Nevertheless, further studies are required to confirm this association.
Conflict of interest
The authors declared no conflict of interest.
References
[1]. Coyle YM. The effect of environment on breast cancer risk. Breast Cancer Res Treat. 2004;84(3):273-88.
[2]. Lux MP, Fasching PA, Beckmann MW. Hereditary breast and ovarian cancer: review and future perspectives. J Mol Med (Berl). 2006;84(1):16-28.
[3]. Huijts PE, Vreeswijk MP, Kroeze-Jansema KH, Jacobi CE, Seynaeve C, Krol-Warmerdam EM, et al. Clinical correlates of low-risk variants in FGFR2, TNRC9, MAP3K1, LSP1 and 8q24 in a Dutch cohort of incident breast cancer cases. Breast Cancer Res. 2007;9(6):R78.
[4]. Mao CF, Qian WY, Wu JZ, Sun DW, Tang JH. Association between the XRCC3 Thr241Met polymorphism and breast cancer risk: an updated meta-analysis of 36 case-control studies. Asian Pacific journal of cancer prevention: APJCP.2014;15(16):6613.
[5]. Sanjari Moghaddam A, Nazarzadeh M, Sanjari Moghaddam H, Bidel Z, Keramatinia A, Darvish H, et al. XRCC1 Gene Polymorphisms and Breast Cancer Risk: A Systematic Review and Meta- Analysis Study. Asian Pac J Cancer Prev. 2016;17(S3):323-30.
[6]. Jiang Y, Han J, Liu J, Zhang G, Wang L, Liu F, et al. Risk of genome-wide association study newly identified genetic variants for breast cancer in Chinese women of Heilongjiang Province. Breast Cancer Res Treat. 2011;128(1):251-7.
[7]. Latif A, Hadfield KD, Roberts SA, Shenton A, Lalloo F, Black GC, et al. Breast cancer susceptibility variants alter risks in familial disease. J Med Genet. 2010;47(2):126-31.
[8]. Tamimi RM, Lagiou P, Czene K, Liu J, Ekbom A, Hsieh CC, et al. Birth weight, breast cancer susceptibility loci, and breast cancer risk. Cancer Causes Control. 2010;21(5):689-96.
[9]. Easton DF, Pooley KA, Dunning AM, Pharoah PD, Thompson D, Ballinger DG, et al. Genome-wide association study identifies novel breast cancer susceptibility loci. Nature. 2007;447(7148):1087-93.
[10]. Gorodnova TV, Kuligina E, Yanus GA, Katanugina AS, Abysheva SN, Togo AV, et al. Distribution of FGFR2, TNRC9, MAP3K1, LSP1, and 8q24 alleles in genetically enriched breast cancer patients versus elderly tumor-free women. Cancer Genet Cytogenet. 2010;199(1):69-72.
[11]. Barnholtz-Sloan JS, Shetty PB, Guan X, Nyante SJ, Luo J, Brennan DJ, et al. FGFR2 and other loci identified in genome-wide association studies are associated with breast cancer in African-American and younger women. Carcinogenesis. 2010;31(8):1417-23.
[12]. Handoll HH. Systematic reviews on rehabilitation interventions. Archives of physical medicine and rehabilitation. 2006;87(6):875.
[13]. Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719-48.
[14]. DerSimonian R, Laird N. Meta-analysis in clinical trials Control Clin Trials. Find this article online. 1986; 7(3):177-88.
[15]. Egger M, Smith GD, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315(7109):629-34.
[16]. Butt S, Harlid S, Borgquist S, Ivarsson M, Landberg G, Dillner J, et al. Genetic predisposition, parity, age at first childbirth and risk for breast cancer. BMC research notes.2012;5:414.
[17]. Campa D, Kaaks R, Le Marchand L, Haiman CA, Travis RC, Berg CD, et al. Interactions Between Genetic Variants and Breast Cancer Risk Factors in the Breast and Prostate Cancer Cohort Consortium. Journal of the National Cancer Institute. 2011;103(16):1252-63.
[18]. Chen Y, Shi C, Guo Q. TNRC9 rs12443621 and FGFR2 rs2981582 polymorphisms and breast cancer risk. World J Surg Oncol. 2016;14(1):50.
[19]. Deng Z, Yang H, Liu Q, Wang Z, Feng T, Ouyang Y, et al. Identification of novel susceptibility markers for the risk of overall breast cancer as well as subtypes defined by hormone receptor status in the Chinese population. Journal of Human Genetics. 2016;61(12):1027-34.
[20]. Mizoo T, Taira N, Nishiyama K, Nogami T, Iwamoto T, Motoki T, et al. Effects of lifestyle and single nucleotide polymorphisms on breast cancer risk: a case-control study in Japanese women. BMC Cancer. 2013;13:565.
[21]. Shan J, Mahfoudh W, Dsouza SP, Hassen E, Bouaouina N, Abdelhak S, et al. Genome-Wide Association Studies (GWAS) breast cancer susceptibility loci in Arabs: susceptibility and prognostic implications in Tunisians. Breast Cancer Res Treat. 2012;135(3):715-24.
[22]. Sueta A, Ito H, Kawase T, Hirose K, Hosono S, Yatabe Y, et al.A genetic risk predictor for breast cancer using a combination of low-penetrance polymorphisms in a Japanese population.Breast Cancer Research and Treatment. 2012;132(2):711-21.
[23]. Tan T, Zhang K, Chen W. Genetic variants of ESR1 and SGSM3 are associated with the susceptibility of breast cancer in the Chinese population. Breast Cancer. 2017;24(3):369-74.
[24]. Sanjari Moghaddam A, Nazarzadeh M, Noroozi R, Darvish H, Mosavi Jarrahi A. XRCC1 and OGG1 Gene Polymorphisms and Breast Cancer: A Systematic Review of Literature. Iran J Cancer Prev. 2016;9(1):e3467.
[25]. Chen MB, Li C, Shen WX, Guo YJ, Shen W, Lu PH. Association of a LSP1 gene rs3817198T>C polymorphism with breast cancer risk: evidence from 33,920 cases and 35,671 controls. Mol Biol Rep. 2011;38(7):4687-95.
[26]. Tang J, Li H, Luo J, Mei H, Peng L, Li X. The LSP1 rs3817198 T > C polymorphism contributes to increased breast cancer risk: a meta-analysis of twelve studies. Oncotarget. 2016;7(39):63960-7.
License
Copyright
© ,
Author Details