Selective Toxicity of Non-polar Bioactive Compounds of Sea Cucumber (Holothuria sabra) Extracts on Isolated Mitochondria and Hepatocytes of Induced Hepatocellular Carcinoma Rat Model
Download
Abstract
objective: Hepatocellular carcinoma (HCC) is the fifth most malignant of liver cancer globally. Anti-inflammatory and anti-cancer properties, scientists have begun to further investigate the natural bioactive compounds found in marine animals. Holothuria sabra (H. sabra), a sea cucumber is known to show the mentioned properties.
method: This study examined the selective toxicity of different dilutions of polar and non-polar extracts (n-hexane, diethyl ether, methanolic and aqueous) obtained from H. sabra, on hepatocytes and isolated mitochondria obtained from hepatocellular carcinoma induced rats. In order to induce HCC on rats, diethyl nitrosamine (DEN) was injected followed by 2-acetylaminofluorene (2-AAF). Finally, hepatocytes and mitochondria isolated from cancerous and normal hepatocytes were applied for subsequent investigations.
Results: Our results show that different concentrations (250, 500 and 1000µg/ml) of the n-hexane, diethyl ether and methanolic extracts significantly (p<0.05) induce reactive oxygen species (ROS) formation and mitochondrial swelling, decreased mitochondrial membrane potential (MMP) disruption, increased cytochrome c release and induced the cell apoptosis phenotypes only in HCC hepatocytes and mitochondria in a time-and concentration dependent manner.
Conclusion: Our results suggest that bioactive compounds found in H. sabra can potentially serve as anti-HCC molecules if further studies such as molecular identification, confirmatory in vivo experiments and clinical trials receive satisfactory results.
Introduction
Hepatocellular Carcinoma (HCC) is categorized among the deadliest cancers and the fifth principal cause of cancer across the world [1, 2]. Causative factors of HCC include Hepatitis C, which has annually increased the rate of HCC up to 3.7 per 100 patients. Hepatitis B has also increased the rate to 2.2 per 100 patients per year [3]. Additional causative factors include food additives, non-alcoholic fatty liver disease, obesity, and various forms of pollutions and industrial chemical toxics found in the environment [4, 5].
Current therapies such as chemotherapy, surgery, and liver transplantation have not been successful treatments given their low efficiency, high recurrence rate, and metastasis [1]. Therefore, an urgent need exists to identify alternative methods for treating various cancers. More than 300 patents have been published within the decade exploring the effects of sea products on cancer therapy [6]. According to the research, approximately 60% of drugs used for cancer treatments have been obtained from natural products [7, 8, 9]. Among them, immense diversity of active compounds have been isolated from marine animals and plants [(10)]. In the past decade, natural marine products have been considered as one of the most promising anti-cancer, anti-tumoral, and anti-inflammatory sources.
The sea cucumber is a popular natural marine animal that resides within deep hot waters. It is an invertebrate marine animal of Holothuroids (Holothuroidea). Prominent characteristics of the marine animal include anti-tumoral and anti-inflammatory properties. Sea cucumbers have been used as a source of food among Asian countries (China, Korea and Taiwan) because of its nutritious and medicinal properties [11, 12].In developed countries (US and Canada), the marine animal has been dried and encapsulated as nutraceuticals for inflammatory conditions in humans and animals [3] .
Although the identification and isolation of bioactive compound extracts of marine animals are within the preliminary stages in comparison to terrestrial dwellers, several forms of the mentioned extracts are undergoing more investigations in clinical trials and are being used as anti-disease drugs. The aim of this is to identify the toxicity effect of polar and non-polar extracts (n-hexane, diethyl ether, methanolic and aqueous extracts) of Holothuria sabra on the cancerous hepatocyte mitochondria, and also on the cancerous hepatocytes of induced HCC rat models, in order to promote further research.
Materials and Methods
Preparation of Holothuria sabra Extraction
Holothuria sabra samples were gathered from deep hot water regions of the Persian Gulf in the province of Hormozgan, south of Iran. Samples were prepared at the Persian Gulf and Oman Sea Ecological Research Institute, Bandar Abbas, Iran. Different forms of extractions: methanolic, n-hexinaic, diethyl ether, and aqueous extracts, were prepared according to the methods described by Sarhadizadeh et al., (2014) [14)]. Bioactive compounds were extracted based on their polarity by using water and different organic solvents. To this reason, sea cucumber samples were washed by distilled water then cut into small pieces, homogenized by blender, and finally, suspended in mentioned extracts for 72 h at room temperature. Subsequently, the suspension was centrifuged for 15 minutes (30,000×g, 4°C) and evaporated by a rotary evaporator at 45°C under vacuum. The obtained powder was stored at -20 °C.
Standardization of extracts by GC-MS analysis
The different extracts of H. sabra were analyzed by GC-MS (Agilent7000 series Triple Quad GC/MS Main Frame). The 30 mm, 0.25 mm, 0.5 mm AB-35MS fused silica capillary column was used as the GC column dimension. This experience was analyzed under the following conditions: injector temperature was 250°C, column temp isothermal was 100°C increased to 250°C at 6°C/min and held at this given temperature for 10 minutes, the ion source temperature was 200°C and the interface temperature was 250°C. In addition, helium gas was engaged as the carrier gas at the rate of 1ml/min. Spectra was obtained in the EI mode with 70eV ionization energy. Finally, the compounds were identified by comparison with the standards.
Animals
Male Sprague-Dawley rats (120 – 130g) purchased from the Institute Pasteur (Tehran, Iran) were kept in air-controlled room at 20 - 25°C; in humidity of 50% - 60%, and for 12 hours daylight. All experiments were accomplished according to the ethical standards of the Committee of Animal Experimentation of Shahid Beheshti University of Medical Sciences in Tehran, Iran. The rats were divided into two groups, healthy rats and induced rats, (A) healthy rats as a control group and (B) rats induced with Hepatocarcinogenesis by a single intraperitoneal (i.p.) injection of DEN (a single dose of 200mg/kg body wt.). After two weeks 2-acetylaminofluorene (2-AAF 0.02%, w/w) was injected every 2 weeks to group B, in order to promote cancer [1, 15].
Cancer Diagnosis Tests
In order to determine the induction of cancer, several experiments were executed. Serum alpha-fetoprotein (AFP) as a blood levels of liver cancer specific marker, liver function tests and liver histopathology of treated rats were examined. The ADVIA Centaur AFP bioassay (Siemens, Germany) was used to determine the concentration of Serum alpha-fetoprotein (AFP). Moreover, alkaline phosphatase (ALP), Serum alanine transaminase (ALT), and aspartate transaminase (AST) as liver enzymes were tested by Hi-tachi-912 chemistry analyzer (Mannheim, Germany) and by standard diagnostic kits (Roche Diagnostics). In addition, a histopathological evaluation as a complementary test was assayed.
Isolation of the Mitochondria from Hepatocytes
Hepatocytes were isolated by using the four-step collagenase liver perfusion method. After that, to examine the cell viability, trypan blue exclusion test was used. For mitochondrial parameters assay, the mitochondria was prepared and extracted from hepatocytes. Briefly, the hepatocytes were suspended in 10mL of solution A (0.25M of sucrose, 0.01M of tricine, 1mM of EDTA, 10mM of NaH2PO4, and 2mM of MgCl2; pH=8) and subsequently frozen at –80°C for 10 min and centrifuged at 760 g for 5 min in order to break the plasma membrane. The supernatant was kept while the pellet was homogenized for 10 min, followed by centrifugation at 760 g for 5 min. The supernatants from the two previous steps were combined and centrifuged for 20 min at 8,000 g. The final mitochondria containing pellet was suspended in Tris buffer (0.05M of Tris-HCl, 0.25M of sucrose, 20 mM of KCl, 2.0mM of MgCl2, and 1.0mM of Na2HPO4; pH=7.4) at 4°C for further tests [1, 16-18].
Succinate Dehydrogenase (SDH) Activity Assay
The activity of mitochondrial complex II, SDH activity, was determined by using a MTT test. In order to do this assessment, 100μl of mitochondrial suspension was incubated with different concentrations of the mentioned polar and non-polar extracts of H. sabra (10, 25, 50,100, 250, 500 and 1000µg/ml) at 37°C for 1 hour. Then, 0.4% of MTT was added to the above suspensions and incubated for 30 min at the previous situation. Finally, 100μl DMSO (5%) was added and the absorbance at 580 nm was measured by an ELISA reader (Tecan, Rainbow Thermo, Austria) [19, 20].
Determination of Reactive Oxygen Species (ROS)
At first, the mitochondrial suspension from both group of animals (HCC induced and normal) were suspended in respiration buffer (20mM of Mops, 10mM of Tris, 0.32mM of sucrose, 0.5mM of MgCl2, 0.1mM of KH2PO4, 5mM of sodium succinate, and 50μM of EGTA). After that, DichlorofluorescinDiacetate (DCFH-DA, at final concentration=10μM) was added then, incubated for 10 minutes at 37°C to measure the ROS level induced by H. sabra extracts. Finally, to determine the intensity of fluorescence induced by dichlorofluorescein (DCF), the Shimadzu RF-5000 U fluorescence spectrophotometer (EX=488 nm and EM=527 nm) was used [21, 22].
Mitochondrial Membrane Potential Assay (MMP)
The cationic fluorescent dye (Rhodamine123, Rh 123) was used to determine the amount of uptake of mitochondrial membrane potential (MMP). Rh 123 (at final concentration=10μM) was added to the mitochondrial suspensions obtained from HCC and normal groups (1000μg mitochondrial protein/ml) in the MMP assay buffer including 2mM of MgCl2, 10mM of KCl, 5mM of KH2PO4, 68 mM of D-mannitol, 50 μM of EGTA, 5 mM of sodium succinate, 10mM of HEPES, 220 mM of sucrose and 2μM of rotenone. Then, the amount of uptake was determined by a Shimadzu RF5000U fluorescence spectrophotometer which was set at EX=490 nm and EM=535 nm [20, 21, 23].
Determination of Mitochondrial Swelling
The isolated mitochondria was suspended in swelling buffer (70mM of sucrose, 230mM of manitol, 3mM of HEPES, 2mM of Tris-phosphate, 5mM of succinate, and 1μM of rotenone) and incubated at 30°C with different concentrations of various forms of non-polar (n-hexane (250, 500 and 1000µg/ml), diethyl ether (250, 500 and 1000µg/ml)) and both polar and non-polar (methanolic (250, 500 and 1000µg/ml)) extracts. The absorbance was determined at 15 min interval, by an ELISA reader (Tecan, Rainbow Thermo, Austria) at 540 nm [22, 24, 25].
Effect of H. sabra on the Cytochrome C Release
Different extracts of H. sabra at IC50 μg/ml (half-maximal inhibitory concentration) induced a significant (P<0.05) release of cytochrome c of the mitochondria isolated from the HCC group. The release of cytochrome c acquired by the mentioned extracts was assayed by the Quantikine Rat/Mouse Cytochrome c Immunoassay kit provided by R&D Systems, Inc. (Minneapolis, MN, USA).
Assessment of Holothuria sabra toxicity on hepatocytes
To evaluate the selective cytotoxicity effect of different extracts of H. sabra on the viability of HCC and normal hepatocytes, MTT (3-(4, 5-dimethylthiazol-2-yl)-2, 5- diphenyl tetrazolium bromide) assay was used. Hepatocytes obtained from normal and HCC cells (1×104 cells/well) were treated with different concentrations (10-1000µm) of non-polar extracts of H. sabra (n-hexane and diethyl ether) and also with polar and non-polar extract (methanolic extract) and incubated in 96-well plates for 90 min, at 37°C in a humidified incubator. Subsequently, MTT (5 mg/ml in RPMI 1640 to each well) was added to the above obtained hepatocytes which were maintained in RPMI 1640, supplemented with 10% FBS and antibiotics (50 U/ml of penicillin and 50µg/ml streptomycin) and then incubated. In the next step, centrifugation began at 1800xg for 5 min at 4°C. The buffer solution (containing different extracts) was inserted into each well and 100 µl of DMSO was then added to dissolve the formazan crystals. Finally, the absorbance was measured at 570 nm by an ELISA reader. Each extract concentration was assessed in three separate experiments (n=3).
Quantification of apoptosis
The percentage of apoptosis versus necrosis induced on hepatocytes obtained from both normal and HCC groups following IC50 μg/ml treatment of n-hexane and diethyl ether (contain non-polar compounds) and methanolic (contains both polar and non-polar compounds) extracts of H. sabra was determined by flow cytometry (equipped with a 605 nm argon ion laser, the flowing software1.2.5. and using a 530nm band pass filter (FL-2 channel)). Before hand, the given cells were seeded over night in 12-well culture plate (75000cells/well) and treated with the mentioned extracts for 90 min. Then, floating and adherent cells were incubated with 750 µL of a hypotonic buffer (50µg/mL propidium iodide (PI) in 0.1% sodium citrate containing 0.1% triton X-100) at 4ºC and under dark conditions. Each determination is based on the mean fluorescence intensity of 10,000 counts.
Statistical Analysis
Graph pad Prism software (version 5) was utilized for all statistical analyses. One-way ANOVA test with post hoc Tukey test was used. Two-way ANOVA test, followed by post hoc Bonferroni test was performed in certain experiments as well. The assays were performed five times (n=5), and results were presented as mean ± SD. Statistical significance was set at P<0.05.
Results
Effect of DEN/2-AAF on liver function markers and AFP
A significant (P<0.05) increase in serum ALT, AST, and ALP (liver function markers), as well as AFP (liver marker for HCC exists in bloodstream) concentrations in HCC group were shown in Tabl 1.
| Group | ALT (IU/L) | AST (IU/L) | ALP (IU/L) | AFP (IU/L) |
| Control group (A) | 93±8.5 | 67±4.8 | 618±8.5 | 0.463±0.02 |
| HCC group (B) | 342± 17*** | 627± 54*** | 778± 10*** | 2.316± 0.09* |
a) Values are presented as mean ± SD (n=9). P < 0.001 compared with group B.
GC-MS Analysis
The GC-MS analysis has shown that, the non-polar extracts of mentioned marine animal reveal the presence of triterpenoids, steroids and dihydrotestosterone according to the Tabl 2. According to this table, the n-hexane extract including, Squalene (12%), Ambrein (15%), β-amyrin (9%), diethyl ether extract including, Renanolone (28%), Lupeol (8%), Drostanolone (15%), and methanolic extract including Lanosterol (14%).
| Extracts | Components | percentages | Structure chemical | Molecular formula | MW |
| N-hexane | Squalene | 25% | Triterpenoids | C30H50 | 410.718 |
| Ambrein | 18% | Triterpenoids | C30H52O | 428.75 | |
| β-amyrin | 19% | Triterpenoids | C30H50O | 426.72 | |
| Diethyl ether | Renanolone | 28% | Steroids | C20H30O4 | 334.450 |
| Lupeol | 18% | Triterpenoids | C30H50O | 426.72 | |
| Drostanolone | 10% | Dihydrotestostrone | C20H32O2 | 304.46 | |
| Methanol | Lanosterol | 24% | Triterpenoids | C30H50O | 426.71 |
Effect of Holothuria sabra extracts on SDH (Succinate Dehydrogenase) Activity
The effects of different concentrations (10, 25, 50,100, 250, 500 and 1000µg/ml) of different extracts of H. sabra on SDH activity of mitochondria obtained from normal and HCC groups were examined by the MTT assay in order to identify the amount of IC50 μg/ml.
Normal and HCC mitochondria were incubated for 1 h in the presence of different concentrations of different extracts of H sabra(10, 25, 50,100, 250, 500 and 1000µg/ml). All applied concentrations of non-polar extracts of H.sabra (n-hexane and diethyl ether) polar and non-polar extract (methanolic extract) indicated a significant (P<0.05) decrease only in the SDH activity of the HCC mitochondria group but did not show any significant effect on normal healthy mitochondria (Fig. 1-2). More importantly, the above result did not occur in the polar extract containing aqueous extract.sabra (10, 25, 50,100, 250, 500 and 1000µg/ml). All applied concentrations of non-polar extracts of H. sabra (n-hexane and diethyl ether) polar and non-polar extract (methanolic extract) indicated a significant (P<0.05) decrease only in the SDH activity of the HCC mitochondria group but did not show any significant effect on normal healthy mitochondria (Fig. 1-2). More importantly, the above result did not occur in the polar extract containing aqueous extract.
Figure 1 : The effect of different concentrations of H sabra on the SDH activity of normal hepatocytes. (A) n-hexane extract, (B) diethyl ether extract, (C) methanolic extract of the H. sabra concentrations and (D) aqueous extract on SDH activity in the mitochondria obtained from hepatocytes. Values are presented as mean ± SD (n = 3). *, ** and *** p<0.05, p<0.01 and p<0.001, respectively, compared to the corresponding control (H. sabra concentration=“0 µg/ml”).sabra on the SDH activity of normal hepatocytes. (A) n-hexane extract, (B) diethyl ether extract, (C) methanolic extract of the H. sabra concentrations and (D) aqueous extract on SDH activity in the mitochondria obtained from hepatocytes. Values are presented as mean ± SD (n = 3). *, ** and *** p<0.05, p<0.01 and p<0.001, respectively, compared to the corresponding control (H. sabra concentration=“0 µg/ml”).
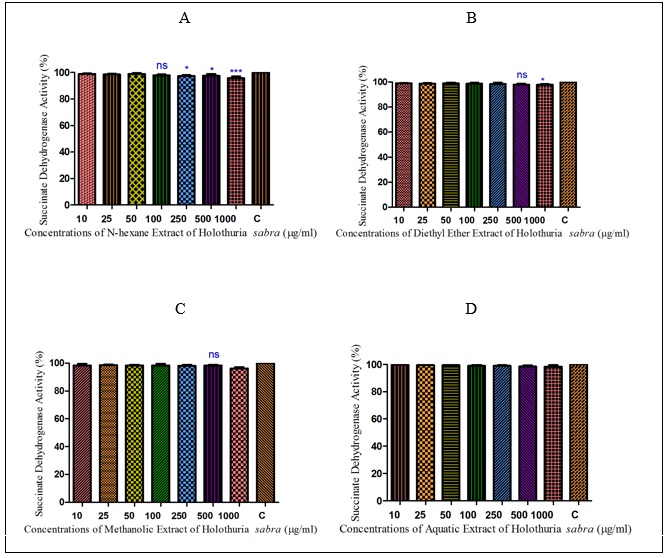
Figure 2 : The effect of different concentrations of H. sabra on the SDH activity of HCC hepatocytes. (A) n-hexane extract, (B) diethyl ether extract, (C) methanolic extract of the H. sabra concentrations and (D) aqueous extract on SDH activity in the mitochondria obtained from HCC hepatocytes. Values are presented as mean ± SD (n = 3). *, ** and *** p<0.05, p<0.01 and p<0.001, respectively, compared to the corresponding control (H. sabra concentration=“0 µg/ml”).
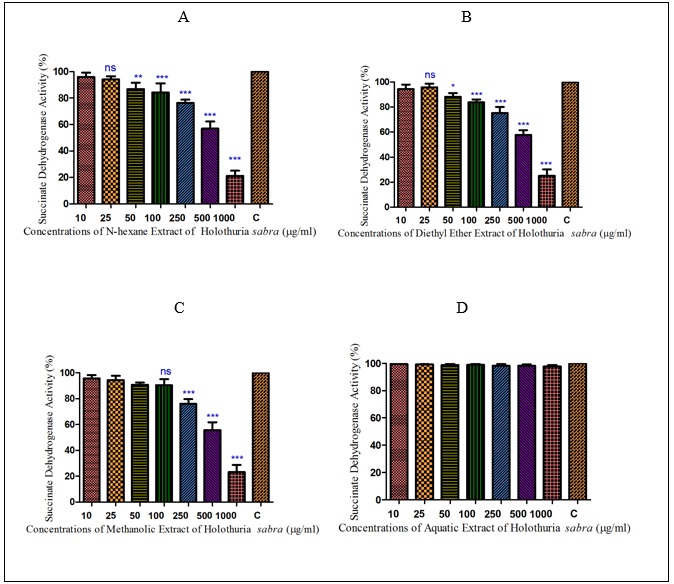
In addition, as shown in (Figure. 2) (A, B), the mentioned non-polar extracts of H. sabra in the highest concentrations (1000µg/ml) significantly (P<0.05) reduced the activity of complex II in the HCC mitochondria compare to normal group. Also, the methanolic extract revealed the same trend concentration-dependent result but at a lower rate (Figure. 2C). But the aqueous extract of H. sabra at all applied concentration (10, 25, 50,100, 250, 500 and 1000µg/ml) did not significantly affect SDH activity (Figure. 2D).
Effect of Holothuria sabra on ROS Production
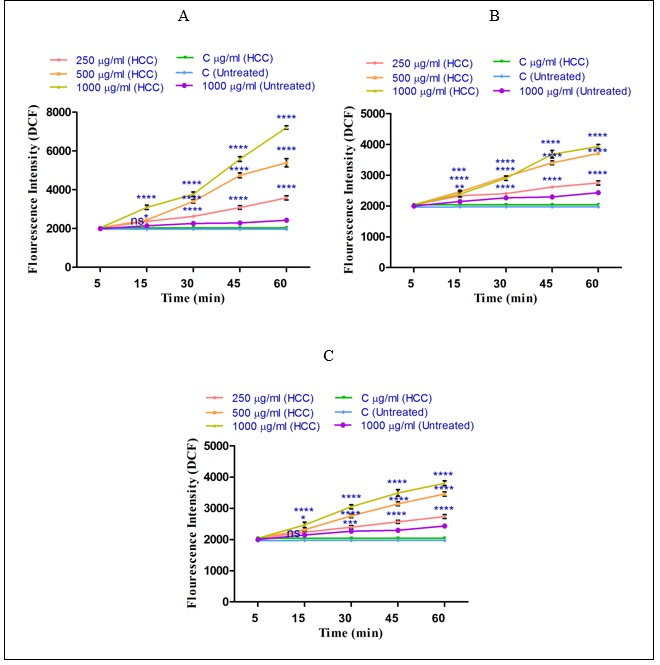
As shown in (Figure 3 A-C) sabrasignificantly (P<0.05) increased ROS generation (demonstrated as fluorescence intensity units emitted from highly fluorescent DCF) in the HCC group. The same result did not occur in the control group. The results of our study show that the n-hexane extract (250, 500 and 1000µg/ml) obtained from the H. sabra on ROS formation is stronger than diethyl ether extract (250, 500 and 1000µg/ml) and methanolic extract (250, 500 and 1000µg/ml) (Fig.3). This activity occurred in a time-and concentration dependent manner. 3 A-C), different concentrations of non-polar extracts (n-hexane and diethyl ether) and both polar and non-polar extract (methanolic extract) of H. sabra significantly (P<0.05) increased ROS generation (demonstrated as fluorescence intensity units emitted from highly fluorescent DCF) in the HCC group. The same result did not occur in the control group. The results of our study show that the n-hexane extract (250, 500 and 1000µg/ml) obtained from the H. sabra on ROS formation is stronger than diethyl ether extract (250, 500 and 1000µg/ml) and methanolic extract (250, 500 and 1000µg/ml) (Fig.3). This activity occurred in a time-and concentration dependent manner.
Figure 3 :The effect of different concentrations of H. sabra on the ROS generation. (A) n-hexane extract (250, 500 and 1000 µg/ml), (B) diethyl ether extract (250, 500 and 1000 µg/ml), (C) methanolic extract (250, 500 and 1000 µg/ml) of the H. sabra concentrations induced a significant increase of mitochondrial ROS formation in HCC but not control mitochondria. Values are presented as mean ± SD (n = 3). *, **, ***and****p<0.05, p<0.01, p<0.001 and p<0.0001, respectively, compared to the corresponding control (H. sabra concentration=“0 µg/ml”).
Effect of Holothuria sabra on Mitochondrial Membrane Potential (MMP)
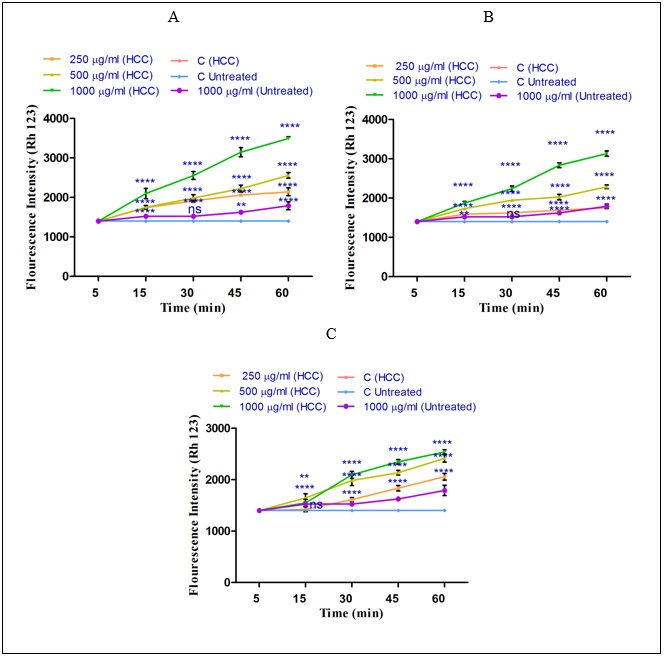
To measure the effect of non-polar and polar extracts of H sabra on MMP, Rh123 staining test was applied. As shown in (Fig. 4) ( A-C), all non-polar (n-hexane and diethyl ether) and both polar and non-polar (methanolic) extracts of H. sabra at different concentrations (250, 500, 1000μg/ml) significantly (P<0.05) raised the absorbance of the fluorescence intensity which determined the collapse of MMP. Actually, the declined MMP demonstrates the distributed damaged mitochondria into the cytosol. According to our results, MMP was significantly (P<0.05) decreased after the addition of the above extracts especially, n-hexane, in a time-and concentration dependent manner by passing 60 min and in a higher concentration. The above results were seen only in the mitochondria obtained from HCC hepatocytes. sabra on MMP, Rh123 staining test was applied. As shown in (Fig. 4) ( A-C), all non-polar (n-hexane and diethyl ether) and both polar and non-polar (methanolic) extracts of H. sabra at different concentrations (250, 500, 1000μg/ml) significantly (P<0.05) raised the absorbance of the fluorescence intensity which determined the collapse of MMP. Actually, the declined MMP demonstrates the distributed damaged mitochondria into the cytosol. According to our results, MMP was significantly (P<0.05) decreased after the addition of the above extracts especially, n-hexane, in a time-and concentration dependent manner by passing 60 min and in a higher concentration. The above results were seen only in the mitochondria obtained from HCC hepatocytes.
Figure 4 : The effect of different concentrations of H. sabra extracts on the MMP collapse. (A) n-hexane extract (250, 500 and 1000 µg/ml), (B) diethyl ether extract (250, 500 and 1000 µg/ml), (C) methanolic extract (250, 500 and 1000 µg/ml) of the H. sabra concentrations induced a significant decrease at MMP in HCC but not control mitochondria. Values are presented as mean ± SD (n = 3). *, **, ***and****p<0.05, p<0.01, p<0.001 and p<0.0001, respectively, compared to the corresponding control (H. sabra concentration=“0 µg/ml”).
Effect of Holothuria sabra on Mitochondrial Swelling
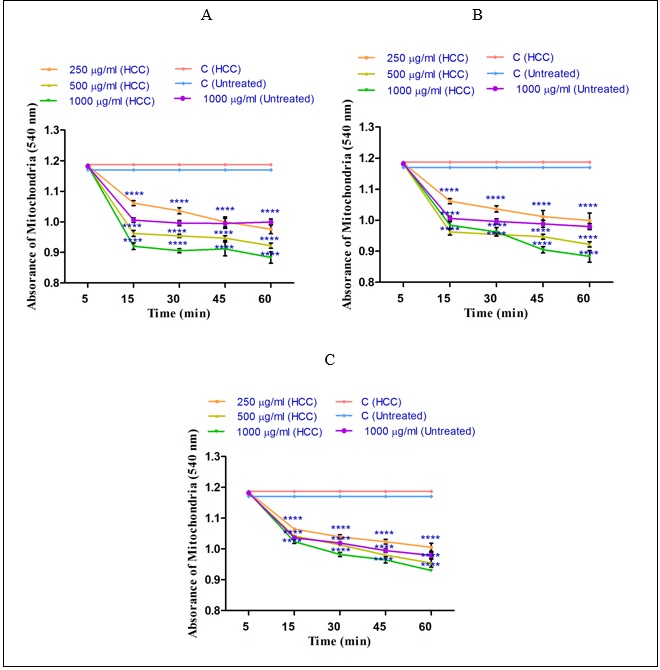
The mitochondrial swelling, as another indicator of the mitochondrial permeability transition (MPT) was assayed. sabra is significant (P<0.05), shown in (Fig. 5). This trend was seen in higher concentration of n-hexane (1000µg/ml), diethyl ether (1000µg/ml) and methanolic (1000µg/ml) extracts of H. sabra in a concentration-and time dependent manner. In addition, the n-hexane extract obtained from H. sabra on mitochondrial swelling is stronger than other extracts (Figure 5A). As shown in (Figure 5 A-C) only the highest applied concentration (1000µg/ml) of all n-hexane, diethyl ether and methanolic extracts significantly influenced mitochondrial swelling of the mitochondria obtained from the normal group.The decrease of absorbance of the mitochondria measured at 540 nm was shown to induce mitochondrial swelling. Increasing rate of mitochondrial swelling of HCC group by the addition of different concentrations of non-polar (n-hexane and diethyl ether (250, 500 and 1000µg/ml) and both polar and non-polar (methanolic (250, 500 and 1000µg/ml)) extracts of H. sabra is significant (P<0.05), shown in (Fig. 5). This trend was seen in higher concentration of n-hexane (1000µg/ml), diethyl ether (1000µg/ml) and methanolic (1000µg/ml) extracts of H. sabra in a concentration-and time dependent manner. In addition, the n-hexane extract obtained from H. sabra on mitochondrial swelling is stronger than other extracts (Figure 5A). As shown in (Figure 5 A-C) only the highest applied concentration (1000µg/ml) of all n-hexane, diethyl ether and methanolic extracts significantly influenced mitochondrial swelling of the mitochondria obtained from the normal group.
Figure 5 :The effect of different concentrations of H. sabra extracts on the mitochondrial permeability. (A) n-hexane extract (250, 500 and 1000 µg/ml), (B) diethyl ether extract (250, 500 and 1000 µg/ml), (C) methanolic extract (250, 500 and 1000 µg/ml) of the H. sabra concentrations induced a significant increase at mitochondrial swelling in HCC but not control mitochondria. Values are presented as mean ± SD (n = 3). *, **, ***and****p<0.05, p<0.01, p<0.001 and p<0.0001, respectively, compared to the corresponding control (H. sabra concentration=“0 µg/ml”).
Effect of Holothuria sabra on the Cytochrome C Release
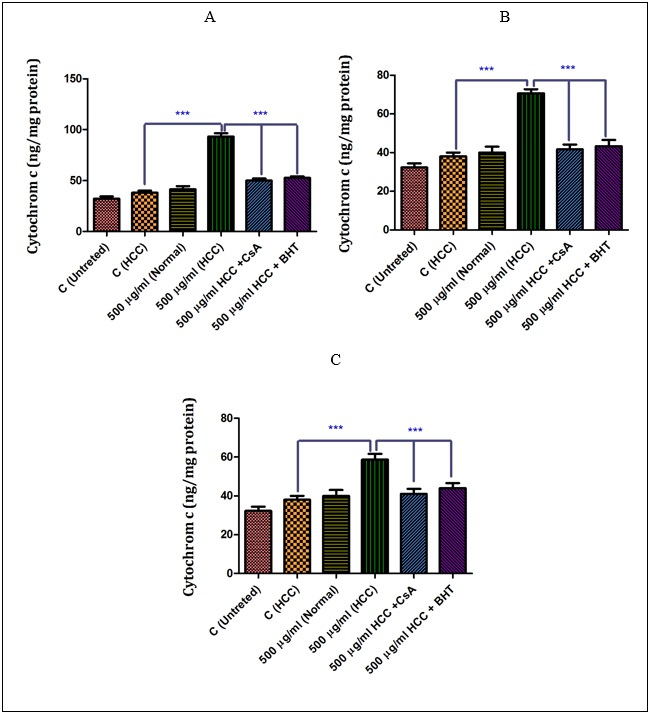
As shown in (Fig A-C),sabra (at IC50 μg/ml) induced a significant (P<0.05) release of cytochrome c only in the mitochondria isolated from the HCC group. Among them, the n-hexane extract showed the highest effect on the HCC group. More importantly, our results show that pretreatment of n-hexane (500 µg/ml), diethyl ether (500 µg/ml) and methanolic extracts (500 µg/ml)-treated mitochondria from the HCC group by cyclosporine A (Cs.A) as a MPT inhibitor, and butylated hydroxyl toluene (BHT) as an antioxidant, inhibited the release of the cytochrome c compared to sole extract of H. sabra treated HCC group without any pretreatments.6)( A-C), the non-polar (n-hexane and diethyl ether) and both polar and non-polar (methanolic) extracts of H. sabra (at IC50 μg/ml) induced a significant (P<0.05) release of cytochrome c only in the mitochondria isolated from the HCC group. Among them, the n-hexane extract showed the highest effect on the HCC group. More importantly, our results show that pretreatment of n-hexane (500 µg/ml), diethyl ether (500 µg/ml) and methanolic extracts (500 µg/ml)-treated mitochondria from the HCC group by cyclosporine A (Cs.A) as a MPT inhibitor, and butylated hydroxyl toluene (BHT) as an antioxidant, inhibited the release of the cytochrome c compared to sole extract of H. sabra treated HCC group without any pretreatments.
Figure 6 :The effect of different concentrations of H. sabra on the cytochrome c release. (A) N-hexane extract (250, 500 and 1000 µg/ml), (B) diethyl ether extract (250, 500 and 1000 µg/ml), (C) methanolic extract (250, 500 and 1000 µg/ml) of the H. sabra significantly increased the cytochrome c release only in HCC but not control mitochondria (P<0.05). Values are presented as mean ± SD (n = 3). *** P<0.001 compared to corresponding control (extract concentration=“0 µg/ml”).
Effect of Holothuria sabra on cell apoptosis
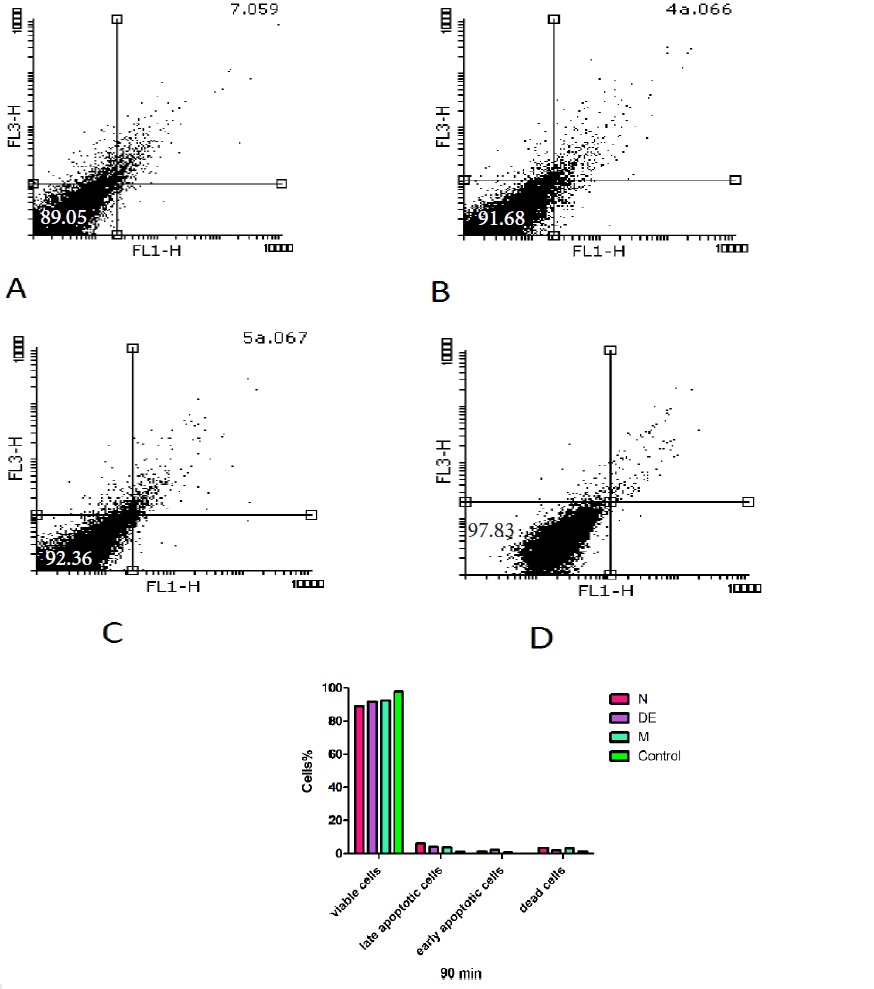
The percentage of apoptosis/necrosis caused by non-polar (n-hexane and diethyl ether) and both polar and non-polar (methanolic) extracts of H sabra was assessed by annexin V-PI double staining at 90 min by flow cytometry. According to the related plots (Fig. 7), the percentage of annexin V+/PI- cells (CD19-gated) significantly (P<0.05) increased following addition of n-hexane > diethyl ether > methanolic extracts of H. sabra on the HCC hepatocytes at IC50μg/ml. This complementary test confirmed the previous results which were induced by mentioned extracts. sabra was assessed by annexin V-PI double staining at 90 min by flow cytometry. According to the related plots (Fig. 7), the percentage of annexin V+/PI- cells (CD19-gated) significantly (P<0.05) increased following addition of n-hexane > diethyl ether > methanolic extracts of H. sabra on the HCC hepatocytes at IC50μg/ml. This complementary test confirmed the previous results which were induced by mentioned extracts.
Figure 7 :The non-polar (n-hexane and diethyl ether) and both polar and non-polar (methanolic) extracts of H. sabra extracts at 500 μg/ml concentration (IC50μg/ml) induced apoptosis in freshly isolated from HCC and normal group. Sum of both early and late apoptotic features were measured by the annexin V assay using flow cytometry at 90 min after incubation in both groups. Results are expressed as means ± SD (n=3), P<0.05.
Discussion
Given the rise of HCC rates globally coupled with inefficient methods for HCC treatment [23], the authors of this paper sought to investigate novel treatment methods and materials to solve this mentioned problem. Marine animals containing various bioactive compounds present strong anticancer and anti-tumor properties [20, 21, 26]. In addition, beyond 70% of anti-cancer agents used, are originally sourced with varieties of lead compounds for different cancer activities [25, 27].
Previous studies have shown that marine animals containing several numbers of functional bioactive compounds significantly induce inhibition of tumor growth rate in a dose dependent manner [28] and suppress tumor viability [21, 26, 29]. Such properties of sea cucumbers are attributed to their bioactive compounds such as triterpenoid glycosides, sulfated polysaccharides and sterols. These bioactive compounds are also popular given their natural origin, long nutritional history and negligible toxic effects. In addition, induction of apoptosis is a prominent anti-cancer mechanism deployed by these compounds [30, 31]. More importantly, this research has shown that a GC-MS of the n-hexane extract of mentioned marine animals reveals the presence of Squalene, Ambrein, β-amyrin, diethyl ether extract reveals Renanolone, Lupeol, Drostanolone and methanolic extract reveals Lanosterol.
This study was designed to discern the screening toxicity of diethyl ether and n-hexane (non-polar), and methanolic extract of both polar and non-polar extracts of H. sabra on HCC isolates from rats.
According to the results of this study, the significant increase in serum markers such as ALT, AST, and ALP (indicators of hepatic dysfunction in the HCC group), illustrate that the liver cancer in rats was induced by DEN/2-AAF. It has been well documented, that increasing levels of the given enzymes in the blood represent liver damage. AFP as another complementary HCC marker increased in HCC-induced rats supporting our hypothesis. Hepatic dysfunction appears to be due to DEN/AAF regimen generating genetic alterations in liver hepatocytes [16, 17, 20, 22, 32, 33, 34]. In addition, histopathological tests reveal HCC hepatocytes with disorganized hepatic lobular architecture and cellular damage to the liver treated by DEN /2-AAF regimen. All of the above findings significantly differ between the HCC group and the control group [23].
Our results show that three extracts of H. sabra at all applied concentrations (250, 500 and 1000μg/ ml) significantly (p<0.05) decrease SDH activity or complex II in the HCC mitochondria in comparison to control rat hepatocytes. On the other hand, the aqueous extract of H. sabra did not have any significant effect on SDH activity.
It has been documented that the mitochondria is the specific source of ROS. Oxygen consumed by the respiratory chain of HCC mitochondria is converted to O2-. Subsequently, superoxide anions generated in the mitochondria are rapidly converted to H2O2; up to 60 to 80% of the cellular generation rate is by the mitochondrial superoxide dismutase (MnSOD). The induced HCC mitochondria (by three extracts of H. sabra), increased the generation of H2O2 formation, and the subsequent and numerous irreversible cellular damages [35].
So, the level of ROS formation as mitochondria parameter toxicity was evaluated. In this study, three extracts of H. sabra at different time and concentration significantly (p<0.05) promoted ROS formation in the mitochondria of the HCC group compared to the control group. Among them n-hexane extract was shown significantly (P<0.05) increase the rate of ROS formation in a time and concentration dependent manner more than the other extracts.
Our findings indicate that the above concentrations of the three extracts of H. sabra significantly induced the collapse of MMP (ΔΨm) in the mitochondria isolated from the HCC group in comparison to the control group. The n-hexane extract of H. sabra significantly reduced the MMP more potently than the other extractions of H. sabra. The collapse of MMP plays a vital role in subsequent processes and apoptosis. Mitochondrial membrane damage can cause MPT pore-opening, release of cytochrome c into the cytosol, which has been resulted in apoptosis and necrosis [36].
Mitochondrial swelling as another indicator of MPT are significantly elevated by the given concentrations of three extracts of H. sabra in the mitochondria obtained from HCC in comparison to the control group (5 A-B). As hypothesized, apoptosis induction is due to oxidative stress, the opening of the MPT pore; the subsequent release of cytochrome c from mitochondria to cytosol after mitochondrial swelling and the collapse of MMP are another disorder pathway.
The most important results were that the mentioned extracts (500µg/ml) of H. sabra significantly induced the release of cytochrome c only from the HCC but not from the control mitochondria.
A defected apoptosis pathway plays a critical role for most malignant tumors, and can be efficient for cancer therapies. Research has shown that apoptosis induced from marine species are due to the presence of bioactive compounds, which are considered as new anti-cancer drug [37].
The distinctive function and characteristic of mitochondria between cancer and normal cells such as the different size, number, and shape of the mitochondria, different structure, and genomic mitochondrial alterations have also been shown in cancer cells in comparison to normal cells [20, 38, 39]. Therefore, the mitochondria was chosen as the main gateway for design and development of anti-cancer drugs [40]. Our results show that non-polar extracts (n-hexane and diethyl ether) of H. sabra (500µg/ml of n-hexane and 500µg/ml of diethyl ether) induce apoptosis in the HCC cell group. The efficacy of sea cucumber extracts at inducing apoptosis of n-hexane was obviously shown in (Fig. 7 A-C). The methanolic extract by both polar and non-polar can induce apoptosis only in the highest concentration (1000 µg/ml).
In conclusion, we suggest that the effects of all extracts of H. sabra on mitochondria and hepatocyte cells may be attributed to the presence of numerous arrays of bioactive compounds such as phenolic, saponins, sterols, cerebrosides and sulfated polysaccharides. As the results show, only non-polar compounds containing extracts can cause selective toxicity alterations in mitochondrial parameters. Hence, we posit non-polar extracts of H. sabra as new anti-HCC drug candidates due to their ability to induce changes in mitochondrial and cellular parameters. In addition, the lower effects of methanolic extract of H. sabra on mitochondria isolated from HCC hepatocytes may be due to the presence of lower non-polar bioactive compounds comparing to n-hexane and diethyl ether extracts. This study provides evidence that H. sabra should be strongly considered as a potentially new anti-HCC drug candidate, as we demonstrate that mitochondrial targeting is a vital mechanism for the Persian Gulf sea cucumber extracts.
Acknowledgments
The results presented in this article were partly extracted from Dr. Nina Seyedrazi’s thesis, Ph.D. graduate of Pharmaceutical Sciences Research Center, Shahid Beheshti University of Medical Sciences, Tehran, Iran. The thesis was performed under the supervision of Prof. Jalal Pourahmad. The investigation was financially supported by research grant (Grant No: 5297) from the Shahid Beheshti University of Medical Sciences, Deputy of Research, Tehran, Iran.
Conflict of Interest
The authors declare no conflict of interest.
References
[1]. Abdel-Hamid N, Abdel-Ghany M, Nazmy M, Amgad S. Can methanolic extract of Nigella sativa seed affect glyco-regulatory enzymes in experimental hepatocellular carcinoma? Environ Health Prev Med. 2013;18(1):49-56.
[2]. Zhang ZY, Hong D, Nam SH, Kim JM, Paik YH, Joh JW, et al. SIRT1 regulates oncogenesis via a mutant p53-dependent pathway in hepatocellular carcinoma. J Hepatol. 2015;62(1):121-30.
[3]. Fattovich G, Stroffolini T, Zagni I, Donato F. Hepatocellular carcinoma in cirrhosis: incidence and risk factors. Gastroenterology. 2004;127(5 Suppl 1):S35-50.
[4]. El Mesallamy HO, Metwally NS, Soliman MS, Ahmed KA, Abdel Moaty MM. The chemopreventive effect of Ginkgo biloba and Silybum marianum extracts on hepatocarcinogenesis in rats. Cancer Cell Int. 2011;11(1):38.
[5]. Burroughs A, Hochhauser D, Meyer T. Systemic treatment and liver transplantation for hepatocellular carcinoma: two ends of the therapeutic spectrum. Lancet Oncol. 2004;5(7):409-18.
[6]. Yalçın F. Biological activities of the marine sponge Axinella. Hacettepe University Journal of the Faculty of Pharmacy. 2007;27(1):47-60.
[7]. Millar RH. The Biology of Ascidians. Advances in Marine Biology. 1971;9:1-100.
[8]. Shenkar N, Swalla BJ. Global diversity of Ascidiacea. PLoS One. 2011;6(6):e20657.
[9]. Millar RH. Ascidians (Tunicata: Ascidiacea) from the northern and north-eastern Brazilian Shelf. Journal of Natural History. 2007;11(2):169-223.
[10]. Jimenez P, Wilke D, Ferreira E, Takeara R, Moraes Md, Silveira Ed, et al. Structure elucidation and anticancer activity of 7-oxostaurosporine derivatives from the Brazilian endemic tunicate Eudistoma vanname. Marine drugs. 2012;10(5):1092-102.
[11]. VSalarzadeh AR, Afkhami M, Bastami KD, Ehsanpour M, Khazaali A, Mokhleci A. Proximate Composition of Two Sea Cucumber Species Holothuria pavra and Holothuria arenicola in Persian Gulf Annals of Biological Research. 2012;3(3):1305-11
[12]. Wijesinghe WAJP, Jeon YJ, Ramasamy P, Wahid MEA, Vairappan CS. Anticancer activity and mediation of apoptosis in human HL-60 leukaemia cells by edible sea cucumber (Holothuria edulis) extract. Food Chemistry. 2013;139(1-4): 326–31.
[13]. Al Marzouqi N, Iratni R, Nemmar A, Arafat K, Ahmed Al Sultan M, Yasin J, et al. Frondoside A inhibits human breast cancer cell survival, migration, invasion and the growth of breast tumor xenografts. Eur J Pharmacol. 2011;668(1-2):25-34.
[14]. Sarhadizadeh N, Afkhami M, Ehsanpour M. Evaluation bioactivity of a sea cucumber, Stichopus hermanni from Persian Gulf. Eur J Exp Biol. 2014;4:254-8.
[15]. Taha MM, Abdul AB, Abdullah R, Ibrahim TA, Abdelwahab SI, Mohan S. Potential chemoprevention of diethylnitrosamine-initiated and 2-acetylaminofluorene-promoted hepatocarcinogenesis by zerumbone from the rhizomes of the subtropical ginger (Zingiber zerumbet). Chem Biol Interact. 2010;186(3):295-305.
[16]. Barogi S, Baracca A, Castelli G, Castelli GP, Bovina C, Formiggini G, et al. Lack of major changes in ATPase activity in mitochondria from liver, heart, and skeletal muscle of rats upon ageing. Mechanisms of ageing and development. 1995;84(2):139-50.
[17]. Seglen PO. Preparation of isolated rat liver cells. Methods Cell Biol. 1976;13:29-83.
[18]. Pourahmad J, Mortada Y, Eskandari MR, Shahraki J. Involvement of Lysosomal Labilisation and Lysosomal/mitochondrial Cross-Talk in Diclofenac Induced Hepatotoxicity. Iran J Pharm Res. 2011;10(4):877-87.
[19]. Zhao Y, Ye L, Liu H, Xia Q, Zhang Y, Yang X, et al. Vanadium compounds induced mitochondria permeability transition pore (PTP) opening related to oxidative stress. J Inorg Biochem. 2010;104(4):371-8.
[20]. Talari M, Seydi E, Salimi A, Mohsenifar Z, Kamalinejad M, Pourahmad J. Dracocephalum: novel anticancer plant acting on liver cancer cell mitochondria. Biomed Res Int. 2014;2014:892170.
[21]. Seydi E, Motallebi A, Dastbaz M, Dehghan S, Salimi A, Nazemi M, et al. Selective Toxicity of Persian Gulf Sea Cucumber (Holothuria parva) and Sponge (Haliclona oculata) Methanolic Extracts on Liver Mitochondria Isolated from an Animal Model of Hepatocellular Carcinoma. . Hepatitis monthly. 2015;15(12):e33073.
[22]. Salimi A, Roudkenar MH, Sadeghi L, Mohseni A, Seydi E, Pirahmadi N, et al. Ellagic acid, a polyphenolic compound, selectively induces ROS-mediated apoptosis in cancerous B-lymphocytes of CLL patients by directly targeting mitochondria. Redox Biol. 2015;6:461-71.
[23]. Razi NS, Seydi E, Nazemi M, Arast Y, Pourahmad J. Selective Toxicity of Persian Gulf Sea Squirt (Phallusia nigra) Extract on Isolated Mitochondria Obtained from Liver Hepatocytes of Hepatocellular Carcinoma Induced Rat. Hepat Mon. 2017;17(2):e41489.
[24]. Shaki F, Hosseini MJ, Ghazi-Khansari M, Pourahmad J. Toxicity of depleted uranium on isolated rat kidney mitochondria. Biochim Biophys Acta. 2012;1820(12):1940-50.
[25]. Hosseini MJ, Shaki F, Ghazi-Khansari M, Pourahmad J. Toxicity of vanadium on isolated rat liver mitochondria: a new mechanistic approach. Metallomics. 2013;5(2):152-66.
[26]. Hussein MR, Haemel AK, Wood GS. Apoptosis and melanoma: molecular mechanisms. J Pathol. 2003;199(3):275-88.
[27]. Zovko A, Viktorsson K, Hååg P, Kovalerchick D, Färnegårdh K, Alimonti A, et al. Marine Sponge Cribrochalina vasculum Compounds Activate Intrinsic Apoptotic Signaling and Inhibit Growth Factor Signaling Cascades in Non–Small Cell Lung Carcinoma. Mol Cancer Ther. 2014;13(12):2941-54.
[28]. V.K.Meenakshi, Paripooranaselvi M, Sankaravadivoo S, Gomathy S, Chamundeeswari KP. Immunomodulatory activity of Phallusia nigra Savigny, 1816 against S-180 IntJCurrMicrobiolAppSci. 2013;2(8):286-95.
[29]. Badaboina S, Bai HW, Park CH, Jang DM, Choi BY, Chung BY. Molecular mechanism of apoptosis induction in skin cancer cells by the centipedegrass extract. BMC Complement Altern Med. 2013;13:350.
[30]. Wang HC, Pao J, Lin SY, Sheen LY. Molecular mechanisms of garlic-derived allyl sulfides in the inhibition of skin cancer progression. Ann N Y Acad Sci. 2012;1271:44-52.
[31]. Janakiram N, Mohammed A, Rao C. Sea Cucumbers Metabolites as Potent Anti-Cancer Agents. Mar Drugs 2015;13(5):2909-23.
[32]. Shen SC, Chen YC, Hsu FL, Lee WR. Differential apoptosis-inducing effect of quercetin and its glycosides in human promyeloleukemic HL-60 cells by alternative activation of the caspase 3 cascade. J Cell Biochem. 2003;89(5):1044-55.
[33]. Ren W, Qiao Z, Wang H, Zhu L, Zhang L. Flavonoids: promising anticancer agents. Med Res Rev. 2003;23(4):519-34.
[34]. Jin D, Anderson E, Gilbert E, Feuerman M. AFP gene expression after acute diethylnitrosamine intoxication is not Afr2 regulated. Cancer Lett. 2005;220(2):211-20.
[35]. Essack M, Bajic VB, Archer JA. Recently confirmed apoptosis-inducing lead compounds isolated from marine sponge of potential relevance in cancer treatment. Mar Drugs. 2011;9(9):1580-606.
[36]. Robertson JD, Orrenius S. Molecular mechanisms of apoptosis induced by cytotoxic chemicals. Crit Rev Toxicol. 2000;30(5):609-27.
[37]. Kozlowski EO, Pavao MS, Borsig L. Ascidian dermatan sulfates attenuate metastasis, inflammation and thrombosis by inhibition of P-selectin. J Thromb Haemost. 2011;9(9):1807-15.
[38]. Lewandowska U, Gorlach S, Owczarek K, Hrabec E, Szewczyk K. Synergistic interactions between anticancer chemotherapeutics and phenolic compounds and anticancer synergy between polyphenols. Postepy Hig Med Dosw (Online). 2014;68:528-40.
[39]. Li M, Miao ZH, Chen Z, Chen Q, Gui M, Lin LP, et al. Echinoside A, a new marine-derived anticancer saponin, targets topoisomerase2alpha by unique interference with its DNA binding and catalytic cycle. Ann Oncol. 2010;21(3):597-607.
[40]. Circu ML, Aw TY. Reactive oxygen species, cellular redox systems, and apoptosis. Free Radic Biol Med. 2010;48(6):749-62.
License
Copyright
© ,
Author Details