Cytotoxic Activity of Some Azole Derivatives
Download
Abstract
Azole derivatives are an important class of compounds in medicinal chemistry with a wide variety of biological activities. We previously described synthesis and antimicrobial evaluations of some new Azole derivatives. Most of our compounds showed desirable activity against different species of microorganisms. Here, we chose seventeen of these compounds, in four different groups including imidazole (group a, 1a-6a), 2-methylimidazole (group b, 1b-4b), 2-methyl-4-nitroimidazole (group c, 1c-4c) and benzimidazole (group d, 1d-3d) to further evaluate their cytotoxic activities against a human cancer cell line (HepG2) in comparison to cisplatin using colorimetric MTT cytotoxic assay. We also compared their cytotoxic activities with clotrimazole to find the safer compounds as antimicrobial agents. Our results indicated that Azole compounds including 2b, 4c, 2d and 3d displayed desirable anti-tumor activities against HepG2 (IC50<50μM) and might be considered as potential anticancer agents for further studies. The o her compounds with less cytotoxicity compared to clotrimazole could introduce as good candidates for antimicrobial agents.
Introduction
Azole compounds have been an interesting source for researchers in the recent century. These derivatives are reported to be physiologically and pharmacologically active and find applications in the treatment of several diseases. Of the Azole compounds, imidazole-based compounds are attractive targets in the design of novel chemical structures for the discovery of new drugs. Imidazole is a 5-membered planar ring containing two basic nitrogen atoms in the 1st and 3rd positions of the Azole ring. Substitution on different positions of this ring provides a number of compounds of interest. Commonly, structure-activity relationship obtained from biological results could be used for further design of new active compounds. Although various azole medications are currently available but toxicity and drug-resistance make the discovery of new active drugs of great importance [1, 2]. Many imidazole analogues have been reported and used as pharmacological agents i.e. azomycine, clotrimazole, miconazole, ergothionine, clonidine and moxonidine.
Although most imidazole-based compounds were first described as antifungal drugs, there are many documents indicating that these drugs i.e. clotrimazole can exert anticancer properties, as well. They usually inhibit mitochondrial-bound glycolytic enzymes and therefore erupt the cycle of energy in cancer cells, decrease cell proliferation, and induce apoptosis [3, 4]. In this regard, in our previous study, we synthetized a variety of new compounds of imidazole and benzimidazole derivatives with potential antifungal and antitumor effects [5 6, ]. We observed that most of our new compounds in particular derivatives of imidazole, 2-methylimidazole, 2-methyl-4-nitroimidazole and benzimidazole, showed desirable antifungal activity [7, 8]. Here, in the present study, we aimed to evaluate the cytotoxicity as well as antitumor activity of seventeen of these compounds on a human hepatocellular carcinoma cell line, HepG2, in comparison to standard drugs, cisplatin and clotrimazole.
Materials and Methods
Chemicals
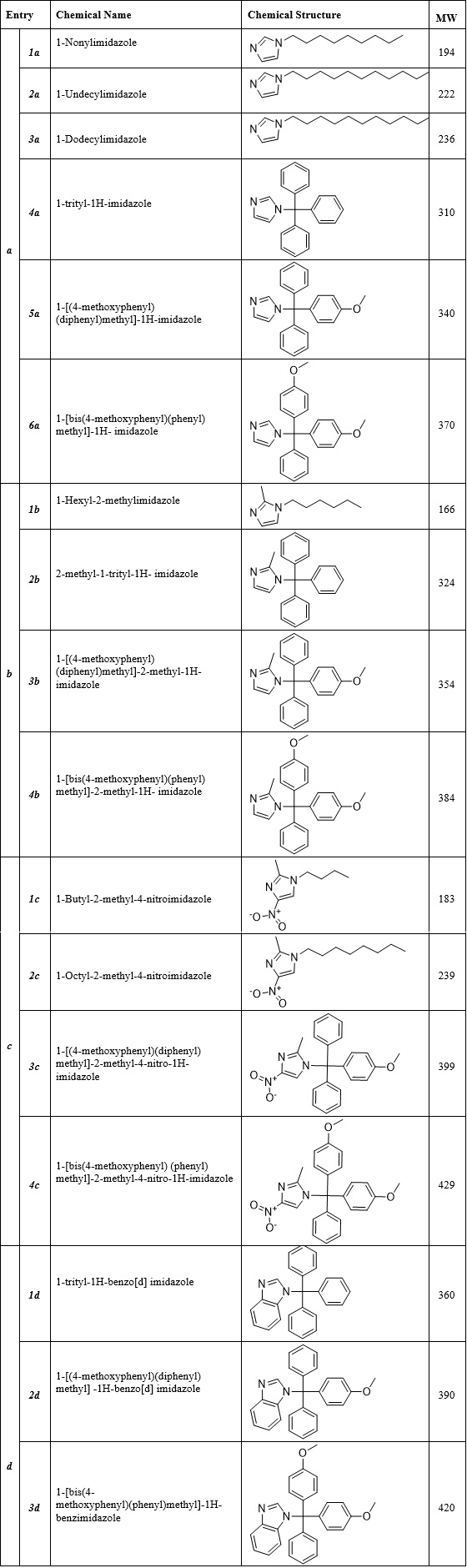
All chemicals and solvents were provided from Merck Company (Germany). Selected synthesized compounds, as illustrated in Table 1 (Fig.1), were provided from our previous study. TLC, melting point and IR spectroscopy were used to assess the purity of the compounds. Clotrimazole was used as positive control.
Figure 1 : Chemical properties of Azole compounds [ 5
Cell Line
Human hepatocellular carcinoma cancer cell line (HepG2) was obtained from the national cell bank of Pasteur Institute of Iran (Tehran, Iran). HepG2 is a cell line derived from a well-differentiated hepatocellular carcinoma of a 15-year-old Caucasian-American male. This line with an epithelial-like morphology, commonly considered as an alternative for the studies on primary human hepatocytes [9].
Cell Culture
Using aseptic techniques, the HepG2 cells were grown in complete culture media containing Roswell Park Memorial Institute (RPMI) 1640 medium, 10% Fetal Bovine Serum (FBS), 100 units/ml penicillin and 100 μg/ml streptomycin (all from Biosera, France), and kept at 37°C in a CO2 incubator with humidified atmosphere. To harvest the cells, following 70-80% of confluency, HepG2 cells were washed and detached using 25% trypsin-EDTA (Biosera, UK). The cells were then washed, counted and prepared for cytotoxic MTT assay as previously described [10, 11].
MTT Cytotoxic Assay
To determine the cytotoxic effects of the Azole compounds, HepG2 cells with the concentration of 10×103/100 µl were seeded per well in 96-well cell culture microplate and left for 24 hours to recover and reattach. The cells were then treated with each compound in different concentrations (1-2000 µM) for 48 hours. Six wells were specified for each concentration, three wells for untreated cells (negative controls), and three wells just included cell culture medium as blanks. Cisplatin and clotrimazole were also used as positive controls. After incubation time, the media were completely discarded and 100µl of MTT reagent (Sigma, Germany) with final concentration of 0.05 mg/ml was added to each well including controls. The plate was incubated for more 3 hours at 37°C till the purple precipitates were appeared. Following removing MTT solution, 100 μl DMSO were added to the wells and leaved in the dark at 37 for 1 hour. All wells’ absorbance was read at 570 nm. Each experiment was separately repeated three times.
Data Analysis
An inhibition concentration, representing the 50% growth inhibition of the cells (IC50), was measured for each compound using Curve Expert 1.5 software. Excel 2013 software package were used for calculations. P values less than 0.05 (two-tailed) were considered statistically significant.
Results and Discussion
Here, we assessed the cytotoxic effects of 17 analogues of our previously synthetized Azole compounds using a colorimetric MTT cytotoxic assay on HepG2, as an in-vitro model for primary human hepatocytes. We first divided the compounds in 4 different groups (a, b, c, d). Group a contains imidazole derivatives (a1-a6). Group b includes 2-methylimidazole derivatives (b1-b4), Group c includes 2-methyl-4-nitroimidazole derivatives (c1-c4) and group d composes of benzimidazole derivatives (d1-d3). The IC50 for each compound are displayed in Table 2. Our results generally showed that however all azole compounds had less cytotoxicity comparing to cisplatin, some of them had comparable IC50 with clotrimazole.
As shown in Tabl 2, the derivatives of imidazole (1a-6a) displayed less cytotoxic effects on HepG2 cells in comparison to clotrimazole. For the linear compounds in this group (1a-3a), the cytotoxic effect enhanced as the length of carbon chain increased. So it could be concluded that longer carbon chain increases the cytotoxicity of imidazole compounds. For the aromatic compounds in this group (4a-6a) cytotoxic effects increased when we have two methoxy substitutions on the trityl ring.
| Compound | IC50 (μM) |
| 1a | 690.68 |
| 2a | 354.5 |
| 3a | 153.11 |
| 4a | 333.6 |
| 5a | 340.39 |
| 6a | 153.74 |
| 1b | 140.45 |
| 2b | 49.01 |
| 3b | 149.31 |
| 4b | 330.59 |
| 1c | 471.06 |
| 2c | 301.23 |
| 3c | 156.46 |
| 4c | 49.46 |
| 1d | 155.8 |
| 2d | 74.4 |
| 3d | 49.89 |
| Clotrimazole | 48.96 |
| Cisplatin | 22.4 |
In group b (1b-4b) all compounds showed less cytotoxic effects on HepG2 cells in comparison to clotrimazole except 2b which showed IC50 near to clotrimazole. In this group, it was observed that adding one methyl group to the imidazole ring significantly increased cytotoxicity of the compounds. These results collectively demonstrate that having methoxy as well as methyl groups simultaneously in the trityl derivatives significantly decreases cytotoxicity.
In case of 2-methyle-4-nitroimidazoles (group c), we observed that adding a nitro substitution on imidazole ring, led to less toxicity except 4c. In this group, similar to group a, increasing the length of carbon chain led to increasing cytotoxic activity. For aromatic compounds in this group it was observed that adding methoxy groups to the trityl ring led to increase cytotoxic effects of the compounds.
In case of trityl benzimidazole series (group d), compound 3d with two methoxy groups was more toxic followed by 2d with one methoxy group and then 1d with no methoxy substitution. However, comparing these compounds to the series a (trityl imidazole); series d because of having benzimidazole instead of imidazole in their structure are more toxic. The increase in the number of methoxy groups also has a synergistic effect on the cytotoxicity of the compounds.
In Conclusion According to the results, among various tested Azole derivatives, 2b, 4c, 2d and 3d are the most cytotoxic compounds against HepG2 cells and can be considered as potential anti-tumor agents for further in-vitro and/or in-vivo studies. Other azole compounds including groups a and b, which displayed less cytotoxicity but had the best antimicrobial activities, as demonstrated in our previous study, could be introduced as suitable candidates for antimicrobial therapy. However, more functional studies are needed to determine the mechanisms of action of the drugs.
Acknowledgments
This work was financially supported by a research Grant No. 93-01-05-7659 provided by the Vice Chancellery of Research of Shiraz University of Medical Sciences, Shiraz, I.R. Iran.
References
[1]. Sharma A, Kumar V, Kharb R, Kumar S, Sharma PC, Pathak DP. Imidazole Derivatives as Potential Therapeutic Agents. Current pharmaceutical design. 2016;22(21):3265-301.
[2]. Shalmali N, Ali MR, Bawa S. Imidazole: An Essential Edifice for the Identification of New Lead Compounds and Drug Development. Mini Rev Med Chem. 2018;18(2):142-63.
[3]. S K, C S, Cs K, S W. Clotrimazole as a Cancer Drug: A Short Review. Medicinal chemistry. 2014;4(11):722-4.
[4]. Sharma GVM, Ramesh A, Singh A, Srikanth G, Jayaram V, Duscharla D, et al. Imidazole derivatives show anticancer potential by inducing apoptosis and cellular senescence. MedChemComm. 2014;5(11):1751-60.
[5]. Khabnadideh S, Rezaei Z, Khalafi-Nezhad A, Pakshir K, Roosta A, Baratzadeh Z. Design and Synthesis of Imidazole and Benzimidazole Derivatives as Antifungal Agents. Anti-Infective Agents in Medicinal Chemistry (Formerly Current Medicinal Chemistry-Anti-Infective Agents). 2008;7(3):215-8.
[6]. Khabnadideh, S. Rezaei, Z. Khalafi-Nezhad, A. Pakshir, K. Heiran. M. J. Shobeiri, H. Design and Synthesis of 2-Methyl and 2-Methyl-4-Nitro-Imidazole Derivatives as Antifungal Agents. Iranian Journal of Pharmaceutical Sciences. 2009;5(1):37-44.
[7]. Khabnadideh S, Rezaei Z, Ghasemi Y, Montazeri-Najafabady N. Antibacterial activity of some new azole compounds. Anti-Infective Agents. 2012;10(1):26-33.
[8]. Khabnadideh S, Rezaei Z, Pakshir K, Zomorodian K, Ghafari N. Synthesis and antifungal activity of benzimidazole, benzotriazole and aminothiazole derivatives. Res Pharm Sci. 2012;7(2):65-72.
[9]. Donato MT, Tolosa L, Gomez-Lechon MJ. Culture and Functional Characterization of Human Hepatoma HepG2 Cells. Methods Mol Biol. 2015;1250:77-93.
[10]. Fereidoonnezhad M, Shahsavari HR, Abedanzadeh S, Behchenari B, Hossein-Abadi M, Faghih Z, et al. Cycloplatinated(ii) complexes bearing 1,1′-bis(diphenylphosphino)ferrocene ligand: biological evaluation and molecular docking studies. New Journal of Chemistry. 2018;42(4);2385-92.
[11]. Fereidoonnezhad M, Shahsavari HR, Lotfi E, Babaghasabha M, Fakhri M, Faghih Z, et al. (Benzyl isocyanide)gold(I) pyrimidine-2-thiolate complex: Synthesis and biological activity. Applied Organometallic Chemistry. 2018;32(3):200.
License
Copyright
© ,
Author Details