Association of rs61803665 Polymorphism in the F11R Gene with Increased Risk of Gastric Cancer
Download
Abstract
Introduction and Objectives: Gastric cancer is one of the most common cancers throughout the world and it is classified as a multifactorial disease. F11R is one of the genes whose role in different cancers is proven. As miR-335-5p and miR-638 are involved in the control of F11R gene expression, and rs61803665 in the F11R gene is located at the binding site to this miRs, we investigated the possible association of this polymorphism with the risk of gastric cancer.
Materials and Methods: 189 gastric cancer patients and 190 healthy individuals were enrolled in this case-control study. Genomic DNA was extracted from blood samples of the patient and the control groups. Using PCR-RLFP technique, the genotype of all samples for rs61803665 in the F11R gene was determined. The results were analyzed statistically using logistic regression and Chi-square tests.
Results: The frequency of genotype AA, AG and GG in the control group were 27.90%, 40.52%, and 31.58%, respectively. The frequency of genotype AA, AG and GG in the patient groups were 24.34%, 30.68%, and 44.98%, respectively. The frequency of allele A and allele G in the control group were 48.15% and 51.85%, respectively. Besides, the frequency of allele A and allele G in the patient groups were 39.68% and 60.32%, respectively. Based on the results of statistical tests, there is a significant correlation between the risk of gastric cancer and genotype GG (P = 0.008, OR = 1.771, % 95CI=1.164-2.693) and allele G (P = 0/019, OR = 1/412, 95% CI = 1/059-1/883).
Conclusion: This study showed that there is an association between allele G at the rs61803665 in the F11R gene and the increased risk of gastric cancer.
Introduction
Gastric cancer is the fourth most common cancer and the second known leading cause of cancer death in the world [1]. Its prevalence in various regions of the world is different, but it is a fundamental problem in developing countries. Similarly, the incidence of gastric cancer in various areas of Iran is different [2][3][4]. It is rarely seen before the age of 40 [5][6]. Despite a decrease in the incidence of gastric cancer in the world, the statistics of the last 30 years show that the incidence of gastric cancer in Iran is higher than the global average [7].
Genetic and environmental factors are involved in Gastric cancer [8]; among the environmental risk factors, the role of Helicobacter pylori infection, lifestyle and nutrition are the most important. Mutations and Genetic polymorphisms are associated with this cancer, also [9][10][11][12]. Most of the genetic diversity in humans result from single nucleotide polymorphisms (SNPs) that cause minor changes in the protein structures or the expression of genes that result in a different response to environmental factors between individuals [13].
The F11R gene is located on the chromosome 1q23.3 encoding the Junctional adhesion molecule-A (JAM-A) protein [14]. This protein plays an important role in the connections of the epithelial and endothelial cells. These plate joints formed around the cells and act as physical barriers, and prevent the free flow of water and soluble materials from intercellular space into the cell [15].
The JAM-A protein is an independent risk factor for the invasiveness of gastric cancer. Lower expression of this factor on the surface of the tumor cells results in the more invasive of tumors and more likely to invade the blood vessel and the lymph nodes, and thus, the tumor is larger and more advanced in tumor stage [16]. There were shown that inappropriate expression of JAM-A protein is associated with the spread and metastasis of gastric cancer [17].
MiRNAs are a group of small regulatory RNAs that can decrease the stability of mRNAs by binding to the un-translational 3’ end region of each mRNA; in this way, they can affect the expression of different genes [18]. Based on the type and activity of the protein in which microRNAs binds to its mRNA and change its expression, they are called tumor suppressor gene or oncogene miRs [19]. It has recently been shown that the expression of mir-335 is impaired in many human cancers, and acts as a tumor suppressor in a variety of tumors [20][21].
Since miR-335-5p and miR-638 play a role in regulating the expression of the F11R gene and rs61803665 is positioned in the binding sites of this miRs, the association of rs61803665 in F11R gene with the risk of gastric cancer is investigated in this study for the first time.
Materials and Methods
In this case-control study, following coordination with Cancer BioBank of Shiraz Institute for Cancer Research (2014-2016) and obtaining written consent from the participants, blood samples were obtained from 189 gastric cancer patients diagnosed by gastroenterologist as well as 190 healthy individuals as the control group that they were matched for gender and age (± 5 years). After DNA extraction by salting out method [22], based on the sequences available on NCBI site and using the OLIGO software (version 5.0; National Bioscience Inc., Plymouth, MN, USA), the following primers were designed for amplification of fragment containing rs61803665 polymorphism:
F- 5'-AGGTGGGCGGATAATGAGGT-3 ', R- 5'TGCCATGGTCAATTAACACATCAC -3'.
Primers were controlled using the NCBI site and Nucleotide-Blast software (www.blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&PAGE_TYPE=BlastSearc h&LINK_LOC = blast home) to ensure the sequence specificity.
The 20 μl PCR reaction mixture included: 12 μl AMPLIQONUSA (Taq Master2x the US), 1 μL of each specific primer pair (20 picomolar), 1.5 μl of genomic DNA and 4.5 μl of double-distilled water.
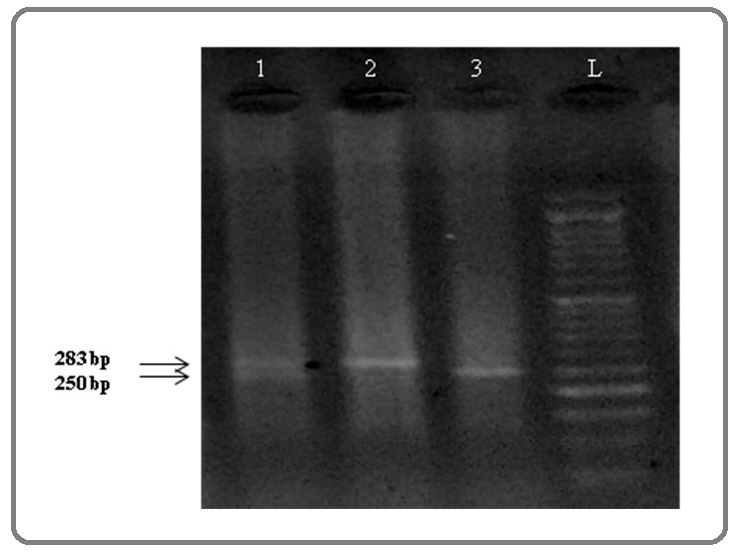
The PCR program included initial denaturation at 94 ° C for 5 minutes, 32 cycles of 94 ° C for the 40s, 57 ° C for 40s and 72 ° C for 40s and the final elongation at 72 ° C for 5 minutes. The accuracy of PCR amplification was confirmed by electrophoresing products on 2% agarose gel. Then, PCR products were treated with the Er1321 restriction enzyme (Germany) according to the kit procedure in a volume of 8 μl included: 3.5 μL of double-distilled water, 0.5 μl of Er1321 enzyme, 0.5 μl 10 X Buffer and 3.5 μl PCR product. Then, it was incubated at 55 ° C for 4 hours. Finally, the products were electrophoresed on 2% agarose gel. Based on the length of the fragments products, the genotypes of all samples were determined (Figure 1).
Figure 1. Enzyme digestion of PCR products and electrophoresis on agarose gel to determint the genotype of rs61803665 polymorphism in the F11R gene. Well 1 genotype AG (280/250/33 bp), Well 2 genotype AA (283 bp), Well 3 genotype GG (250/33 bp). 33 bp band is not visible due to being small.
Statistical analysis
To examine the association between rs61803665 polymorphism in F11R gene and the risk of gastric cancer, the results were statistically analyzed using SPSS package (Version 20.0, SPSS Inc., Chicago, IL, USA), and logistic regression and Chi-square tests, and the level of statistical significance were set at p < 0.05.
Results
The total number of subjects enrolled in this study was 379 individuals including 189 gastric cancer patients and 190 healthy individuals as the control group. The mean age of the patients was 58.86 ± 13.77 years, and in the control group was 59.24 ± 13.93 years. The characteristics of healthy and patient groups are presented in Table 1.
| Control (%n) | Patient (%n) | |
| Number | 190 | 189 |
| Age range | 26-89 | 27-93 |
| Average age (year) | 57/86 ± 13/77 | 59/24 ± 13/93 |
| Male | 59/37± 13/68 | 59/97± 12/92 |
| Female | 55/42 ± 13/67 | 57/92 ± 15/65 |
| Sex | ||
| Male | 119 | 119 |
| Female | 71 | 70 |
| Type of cancer | ||
| Diffuse | - | 81 |
| Intestinal | - | 34 |
| Unknown | - | 74 |
Among controls (χ2=6.75, df=1, p<0.05) and patients (χ2=24.75, df=1, p<0.05) the observed genotype frequencies of the F11R gene polymorphism deviate significantly from those expected from the Hardy–Weinberg equilibrium.
After determining the genotypes of all samples, the frequency of reference allele A in healthy individuals was 48.15% and in patients was 39.68%. Also, the frequency of AA genotype in the control and patient groups were 27.90% and 24.34%, respectively. Similarly, the frequency of G allele in the healthy group was 51.85% and in the patient group were 60.32%, and the frequency of GG genotype in the control and patient groups were 31.58% and 44.98%, respectively. Likewise, the frequency of AG genotype in the control and the patient groups were 40.52% and 30.68%, respectively. The AA genotype at this locus was considered as the reference.
On the other hand, when the AA+AG genotype was considered as the reference, the results of statistical tests showed a significant correlation between the GG genotype and the risk of gastric cancer (P= 0.008, OR= 1.771, 95CI= 1.164-2.693). Also, the results revealed the association of allele G with the risk of this disease (P= 0.019, OR= 1.412, 95% CI= 1.059-1.883) (Table 2).
| Genotype/ Allele | Control (%) | Patient (%) | P value | OR | %95 CI |
| AA | 53 (27.90) | 46 (24.34) | - | 1 | - |
| AG | 77 (40.52) | 58 (30.68) | 0.594 | 0.868 | 0.515-1.462 |
| GG | 60 (31.58) | 85 (44.98) | 0.062 | 1.632 | 0.975-2.732 |
| AA+AG | 130 (68.42) | 104 (55.02) | - | 1 | - |
| GG | 60 (31.58) | 85 (44.98) | 0.008 | 1.771 | 1.164-2.693 |
| Allele A | 183 (48.15) | 150 (39.68) | - | 1 | - |
| Allele G | 197 (51.85) | 228 (60.32) | 0.019 | 1.412 | 1.059-1.883 |
*OR: Odd Ratio, CI: Confidence Interval
Also, when the genotype AA+AG was placed as the reference, the results of statistical tests showed a significant correlation between GG genotype and the women susceptibility to stomach cancer disease (P= 0/003, OR= 2/944, % 95CL= 1.446-5.994). After categorization of patients according to Lauren's classification (diffuse and intestinal), the results indicate that there is no significant relationship between rs61803665 polymorphism in the F11R gene and the risk of the type of gastric cancer (Table 3).
| Diffuse (%) | Genotype | Intestinal (%) | P value | OR | %95 CI |
| AA | 14 (17.29) | 8 (23.52) | - | 1 | - |
| AG | 33 (40.75) | 13 (38.24) | 0.5 | 0.689 | 0.234-2.030 |
| GG | 34 (41.96) | 13 (38.24) | 0.465 | 0.67 | 0.228-1.967 |
*OR: Odd Ratio, CI: Confidence Interval
Discussion
Various studies show that the onset of cancer and its subsequent progression to higher stages are greatly influenced by genetic factors that determine the characteristics of the individual [13].
Cell adhesion molecules are involved in the regulation of important processes, including cell proliferation, differentiation, and morphogenesis. JAM-A is one of these molecules that has a short cytoplasmic tail and lacks catalytic activity. However, it plays a role in various biological processes through reactions with various proteins, and its expression changes in various cancers [23].
In this case-control study, for the first time, we examined the rs61803665 located in miR-335-5p, miR-638 binding site in the F11R gene, encoding JAM-A protein, in patients with gastric cancer. The results of statistical tests showed a significant association between susceptibility to gastric cancer and genotype GG and the allele G. It should be noted that the frequency of genotypes in the patients and control groups were not in Hardy–Weinberg equilibrium that may be due to the small size of our samples.
Various studies have been carried out on the role of the F11R gene in cancers [24]. Min Zhang et al. (2013) investigated JAM-A protein and lung cancer. They concluded that JAM-A protein expression was high in 37% of the patients with lung cancer compared to normal cases [25]. Similarly, Takuya kakuki et al. (2016) studied the association of JAM-A protein and squamous cell carcinoma of head and neck and their possible changes. Their results indicated a high expression of JAM-A protein in head and neck cancer cells. Also, JAM-A protein has a high expression in the invasion and metastasis of lymph nodes. Also, excessive expression of JAM-A protein is associated with metastatic breast, lung and pancreatic cancers [26]. In contrast, Jin-yu Huang et al. (2014) found that decrease in JAM-A protein level in gastric cancer result in tumor invasiveness [17].
Jia-If Zhang et al. (2017) studied the correlation of miR -335 and gastric cancer and concluded that mir-335 is involved in the suppression, metastases, and invasiveness of many different cancers. High level of methylation in the CpG islands regions of miR -335 genes associated with a low level of it gastric cancer tissue. Expression of miR -335 in gastric cancer cells has an important effect on the process of decreasing tumor migration. In general, miR-335 acts as a tumor suppressor. However, the mechanism responsible for expressing this miR in gastric cancer is still unknown [27].
Regarding to rs61803665 is located at the 3 untranslated region of the F11R gene, and this site is the binding site of two miRs (miR-335-5p and miR-638), also the expression of these miRs are altered in various cancers, especially miR-335, which specifically changes in gastric cancer, thus, the results of this study seem to be reasonable.
In conclusion,the results of this study showed that there is an association between rs61803665 polymorphism in F11R gene and increased risk of gastric cancer. Because of its medical importance, it is suggested that this polymorphism be investigated in larger populations, and if the same results are obtained, it could be used as a genetic biomarker in screening for gastric cancer.
References
- Geostatistical Analysis of County-Level Lung Cancer Mortality Rates in the Southeastern United States. 美国东南部县级肺癌死亡率的地统计学分析 Goovaerts Pierre. Geographical Analysis.2010;42(1). CrossRef
- Major Causes of Death among Men and Women in China He Jiang, Gu Dongfeng, Wu Xigui, Reynolds Kristi, Duan Xiufang, Yao Chonghua, Wang Jialiang, Chen Chung-Shiuan, Chen Jing, Wildman Rachel P., Klag Michael J., Whelton Paul K.. New England Journal of Medicine.2005;353(11). CrossRef
- Gastric cancer in Iran: epidemiology and risk factors Malekzadeh R, Derakhshan MH, Malekzadeh Z. Archives of Iranian medicine.2009;12(6):576-583.
- Prevalence of gastric precancerous lesions in Ardabil, a high incidence province for gastric adenocarcinoma in the northwest of Iran Malekzadeh R. Journal of Clinical Pathology.2004;57(1). CrossRef
- Trends of cancer incidence and mortality in Cali, Colombia. 50 years experience Bravo LE, Collazos T, Collazos P, Garcia LS, Correa P. Colombia medica (Cali, Colombia).2012;43(4):246-255.
- Population Attributable Risks of Esophageal and Gastric Cancers Engel L. S., Chow W.-H., Vaughan T. L., Gammon M. D., Risch H. A., Stanford J. L., Schoenberg J. B., Mayne S. T., Dubrow R., Rotterdam H., West A. B., Blaser M., Blot W. J., Gail M. H., Fraumeni J. F.. JNCI Journal of the National Cancer Institute.2003;95(18). CrossRef
- Adenoviral gene therapy in gastric cancer: A review Khalighinejad Nima, Hariri Hesammodin, Behnamfar Omid, Yousefi Arash, Momeni Amir. World Journal of Gastroenterology.2008;14(2). CrossRef
- Multifactorial etiology of gastric cancer Zabaleta J. Methods in molecular biology (Clifton, NJ).2012;863:411-435.
- A Prospective Study of Tobacco, Alcohol, and the Risk of Esophageal and Gastric Cancer Subtypes Freedman N. D., Abnet C. C., Leitzmann M. F., Mouw T., Subar A. F., Hollenbeck A. R., Schatzkin A.. American Journal of Epidemiology.2007;165(12). CrossRef
- Helicobacter pylori and its effects on human health and disease Kamangar F, Sheikhattari P, Mohebtash M. Archives of Iranian medicine.2011;14(3):192-199.
- Evaluation of the 7th UICC TNM Staging System of Gastric Cancer Kwon Sung Joon. Journal of Gastric Cancer.2011;11(2). CrossRef
- Helicobacter pylori Infection in Intestinal- and Diffuse-Type Gastric Adenocarcinomas Parsonnet J., Vandersteen D., Goates J., Sibley R. K., Pritikin J., Chang Y.. JNCI Journal of the National Cancer Institute.1991;83(9). CrossRef
- Searching for polymorphisms that affect gene expression and mRNA processing: Example ABCB1 (MDR1) Wang Danxin, Sadée Wolfgang. The AAPS Journal.2006;8(3). CrossRef
- Identification and characterisation of human Junctional Adhesion Molecule (JAM) Williams L.A, Martin-Padura I, Dejana E, Hogg N, Simmons D.L. Molecular Immunology.1999;36(17). CrossRef
- JAM-A overexpression is related to disease progression in diffuse large B-cell lymphoma and downregulated by lenalidomide Xu Peng-Peng, Sun Yi-Feng, Fang Ying, Song Qi, Yan Zi-Xun, Chen Yi, Jiang Xu-Feng, Fei Xiao-Chun, Zhao Yan, Leboeuf Christophe, Li Biao, Wang Chao-Fu, Janin Anne, Wang Li, Zhao Wei-Li. Scientific Reports.2017;7(1). CrossRef
- The JAM family of junctional adhesion molecules Bazzoni Gianfranco. Current Opinion in Cell Biology.2003;15(5). CrossRef
- Low junctional adhesion molecule A expression correlates with poor prognosis in gastric cancer Huang Jin-yu, Xu Ying-ying, Sun Zhe, Wang Zhen-ning, Zhu Zhi, Song Yong-xi, Luo Yang, Zhang Xue, Xu Hui-mian. Journal of Surgical Research.2014;192(2). CrossRef
- miR-335 Represents an Independent Prognostic Marker in Epithelial Ovarian Cancer Cao Jin, Cai Jing, Huang Da, Han Qing, Chen Yanxia, Yang Qiang, Yang Chun, Kuang Yan, Li Donglin, Wang Zehua. American Journal of Clinical Pathology.2014;141(3). CrossRef
- MicroRNAs, the DNA damage response and cancer Wouters Maikel D., van Gent Dik C., Hoeijmakers Jan H.J., Pothof Joris. Mutation Research/Fundamental and Molecular Mechanisms of Mutagenesis.2011;717(1-2). CrossRef
- MiR-335 Inhibits Small Cell Lung Cancer Bone Metastases via IGF-IR and RANKL Pathways Gong Meng, Ma Junrong, Guillemette Ryan, Zhou Mingliang, Yang Yan, Yang Yujing, Hock Janet M., Yu Xijie. Molecular Cancer Research.2013;12(1). CrossRef
- A simple salting out procedure for extracting DNA from human nucleated cells Miller S.A., Dykes D.D., Polesky H.F.. Nucleic Acids Research.1988;16(3). CrossRef
- Junctional adhesion molecule-A: functional diversity through molecular promiscuity Steinbacher Tim, Kummer Daniel, Ebnet Klaus. Cellular and Molecular Life Sciences.2017;75(8). CrossRef
- Overexpression of JAM-A in Non-Small Cell Lung Cancer Correlates with Tumor Progression Zhang Min, Luo Wenting, Huang Bo, Liu Zihui, Sun Limei, Zhang Qingfu, Qiu Xueshan, Xu Ke, Wang Enhua. PLoS ONE.2013;8(11). CrossRef
- Tight junctions in cancer metastasis Martin TA, Mason MD, Jiang WG. Frontiers in bioscience (Landmark edition).2011;16:898-936.
- Dysregulation of junctional adhesion molecule-A via p63/GATA-3 in head and neck squamous cell carcinoma Kakuki Takuya, Kurose Makoto, Takano Ken-ichi, Kondoh Atsushi, Obata Kazufumi, Nomura Kazuaki, Miyata Ryo, Kaneko Yakuto, Konno Takumi, Takahashi Syunta, Hatakeyama Tsubasa, Kohno Takayuki, Himi Tetsuo, Kojima Takashi. Oncotarget.2016;7(23). CrossRef
- Cancer incidence in Spain, 2015 Galceran J., Ameijide A., Carulla M., Mateos A., Quirós J. R., Rojas D., Alemán A., Torrella A., Chico M., Vicente M., Díaz J. M., Larrañaga N., Marcos-Gragera R., Sánchez M. J., Perucha J., Franch P., Navarro C., Ardanaz E., Bigorra J., Rodrigo P., Bonet R. Peris. Clinical and Translational Oncology.2017;19(7). CrossRef
- Long non-coding RNA NEAT1 regulates epithelial membrane protein 2 expression to repress nasopharyngeal carcinoma migration and irradiation-resistance through miR-101-3p as a competing endogenous RNA mechanism Wang Yujia, Wang Chunting, Chen Can, Wu Fengbo, Shen Pengfei, Zhang Peng, He Gu, Li Xiang. Oncotarget.2017;8(41). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2019
Author Details