Survival Benefit of Transarterial Chemoembolization for Hepatocellular Carcinoma
Download
Abstract
Objective: Transarterial chemo-embolization (TACE) is a palliative treatment option for hepatocellular carcinoma (HCC) with improved patient survival. The aim of the study was to see the outcome of our patients at our institution 2 years post TACE.
Patients and Methods: Electronic records were retrospectively reviewed for patients who had TACE from 1st November 2009 – 31st October 2012. Baseline imaging, multidisciplinary team (MDT) and clinical notes, pathology labs, TACE angiograms and follow up imaging were reviewed for 2 years after first TACE. Procedure complications, clinical status and findings at follow up CT were reviewed and analyzed in SPSS version 19. Survival was assessed using Kaplan Meier curves.
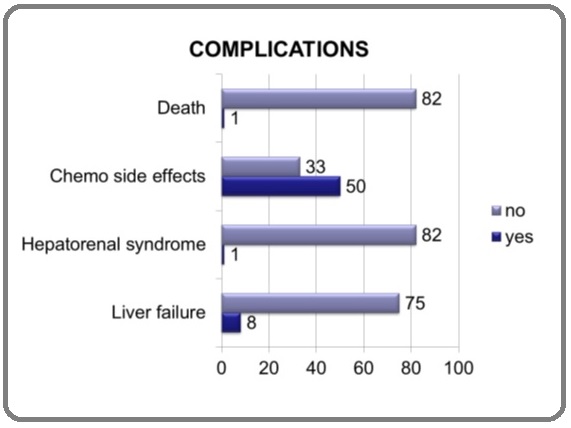
Results: A total of 104 patients had TACE for HCC. Amongst these 21 patients were lost to follow up and could not be contacted to reconfirm the outcome and had to be excluded to minimize bias. Amongst the included 83 patients, 57 (68.7%) were male and 65 (78.4%) were 51-70 years of age at time of first TACE. The commonest cause for HCC was HCV in 74 (89.2%) patients. Target lesion size at baseline CT was < 5 cm in 38 (45.8%) patients, 5-10 cm in 37 (44.6%) patients and >10 cm in 8 (9.6%) patients. A total of 25 (30%) patients needed more than 1 session of TACE. On post TACE CT, 46 (55.4%) patients had good packing of lipoidol in the lesion. A total of 18 (21.7%) patients progressed in TACED lesions while another 21 (25%) progressed with new lesions. One patient had metastasis to adrenal gland. Only 8 (9.6%) patients had liver failure after TACE and 1 patient had hepatorenal syndrome. Only 1 patient died within 30 days after TACE. Two years post TACE, 47 (56.6%) patients were alive indicating good outcome.
Conclusion: TACE improves survival in HCC; 1 year survival was 80% and 2 year survival was 56.6%.
Introduction
HCC is the fifth most frequent cancer in the world and the third most common cause of cancer-related mortality in the world [1][2]. The incidence of hepatocellular carcinoma is rising worldwide due to the HBV and HCV epidemics and the successful treatment of other complications of cirrhosis [3]. The diagnosis is made based on imaging features on multiphasic abdominal CT scan or dynamic MRI imaging and correlating it with serum alpha-feto protein levels. Alpa-feto protein is the only serological marker commonly used in diagnosis, but has a poor sensitivity ranging from 39% to 65% and a specificity ranging from 76% to 97% [4]. In case of atypical imaging features, the lesion is subjected to biopsy. Decision on biopsy of a focal liver lesion should be discussed by the multidisciplinary team, including a hepatobiliary and transplant surgeon. There is a risk of tumor seeding during biopsy varies between 0% and 11%. [5]. Despite the widespread use of screening programs, 60–70% of HCCs are detected when curative treatments (surgery or ablation) are precluded by the cancer burden or contraindications [6].
Among the available curative and palliative treatment options, Transcatheter arterial chemoembolisation (TACE) is the most common and treatment of choice for intermediate stage hepatocellular carcinoma (HCC) [2][7]. TACE includes the selective injection through the hepatic artery of antineoplastic agents (e.g. cisplatin, doxorubicin, mitomycin), together with selective obstruction of tumoral feeding vessels (e.g. with coils, gelatine sponge particles). TACE yields response rates of 35–42% and prolongs life in comparison with best supportive care in selected patients with intermediate advanced HCC (Child-Pugh A, no portal invasion or extrahepatic metastases) [9].
The rationale of TACE is unique dual hepatic blood supply. In contrast to normal and regenerating liver in cirrhosis which gets nearly 80% blood supply from portal vein, HCC receives its blood supply predominantly through the hepatic arterial system. Therefore TAE or TACE has a marked anti-tumoral effect while only minimally affecting the surrounding liver parenchyma.
Materials and Methods
Patient Selection
The retrospective study was conducted in which the electronic records of patients who had TACE from 1st Nov 2009 to 31st Oct 2012 were reviewed. Each patient was selected for TACE after discussion in MDT. Baseline imaging, multidisciplinary team (MDT) and clinical notes, pathology labs, TACE angiograms and follow up imaging were reviewed for 2 years after first TACE. Procedure complications, clinical status and findings at follow up CT were reviewed. Patients who had lost to follow up at 2 years or their family was contacted on phone and inquired about current status. Non-responders were excluded.
Procedure details
Patients underwent TACE from the common femoral artery approach after informed written consent and review of fresh labs. Celiac and superior mesenteric angiograms were obtained to assess arterial anatomy and tumor vasculature. The chemotherapeutic agent (doxorubicin) mixed with lipiodol was than infused into the feeding artery and stasis obtained.
Follow-up
Follow up imaging was performed. Data of clinical notes, pathology labs for monitoring of ascites, hepatic encephalopathy and liver function tests was also reviewed.
Imaging protocol
Pre-treatment CT or MRI scans were used for documenting the initial findings. The radiological requirement required for discussion in the HCC conference was either a biphasic contrast-enhanced CT or dynamic MRI. Local response to therapy was documented 6 weeks after TACE using triphasic CT scan acquiring unenhanced, arterial and portal venous phases. The response to therapy was reassessed by review of imaging by four experienced radiologists having experience of more than 5 years. The basis for this was comparison of the initial finding with the last documented examination of the patient.
Objective
The objective of this study was to assess the overall patient outcome at our Hospital and compare with international standards.
Statistical Analysis
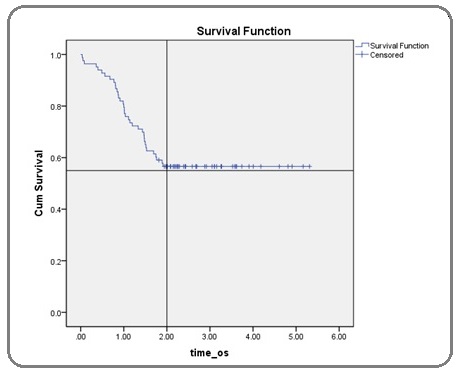
All the collected data entered into SPSS version 17 and analysed. Data was stratified according to age, gender, child Pugh score [8], aetiology, lesion size at baseline, uni or multifocality of lesions, involvement of single or both lobes, selective/non-selective TACE, hepatic artery embolization, number of sessions and lipiodol packing on post-TACE scan. Kaplan-Meir curve was used to estimate survival up to two years (Figure 1).
Figure 1: Kaplan Meier Curve Indicating 1 Year Survival of 80% and 2 Year Survival of 56.6% Post TACE.
Results
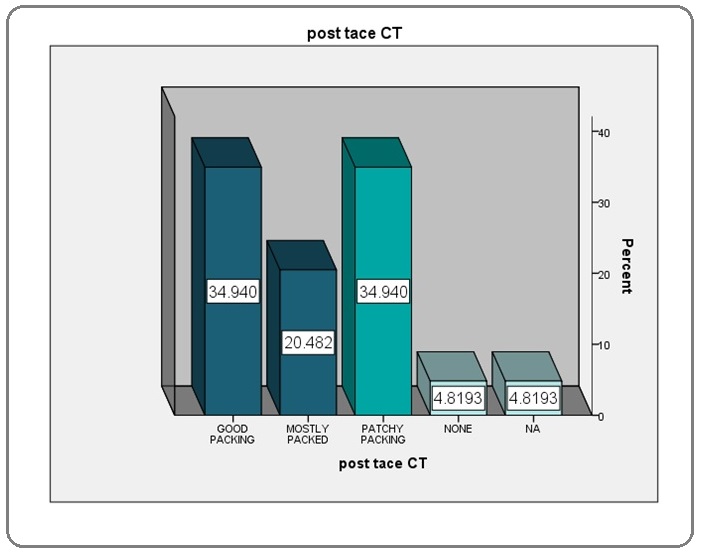
A total of 104 patients had TACE for HCC. Amongst these 21 patients were lost to follow up and even their families could not be contacted to reconfirm the outcome and had to be excluded to minimize bias. Amongst the included 83 patients, 57 (68.6%) were male and 26 (31.33%) were female. Age of 65 patients (78.4%) ranged between 51-70 years at time of first TACE with the mean age of overall study population to be 59.94 years. The commonest cause for HCC was HCV in 74 (89.2%) patients. 78 patients (94%) had Child’s score A while 5 patients (6%) had child’s score B [8]. Target lesion size at baseline CT was <5cm in 38 (45.8%) patients, 5-10 cm in 37 (44.6%) patients and >10 cm in 8 (9.6%) patients. In terms of disease focality; 34 patients (40.96%) had bifocal disease, 30 patients (36.14%) had unifocal disease while 19 (22.89%) had multifocal disease. 59 patients (71.1%) had unilobar disease while both hepatic lobes were involved in 24 patients (28.92%). 77 patients (92.3%) underwent non-selective TACE. Three patients had selective TACE while 3 patients had more than one session which included selective as well as non-selective TACE. Hepatic artery was embolized in 77 patients (92.3%). 58 patients (69.9%) underwent only one session of TACE while 20 patients (24.1%) had two sessions.5 patients (6.01 %) underwent three sessions of TACE.
Lipiodol packing on Post-TACE CT
Patients having over 75% of the lesion packed with lipiodol were labelled to have good packing, between 50-75% were labelled to have mostly packed while those with less than 50% of packing were labelled to have patchy packing (Figure 2).
Figure 2: Lipiodol Packing Percentage.
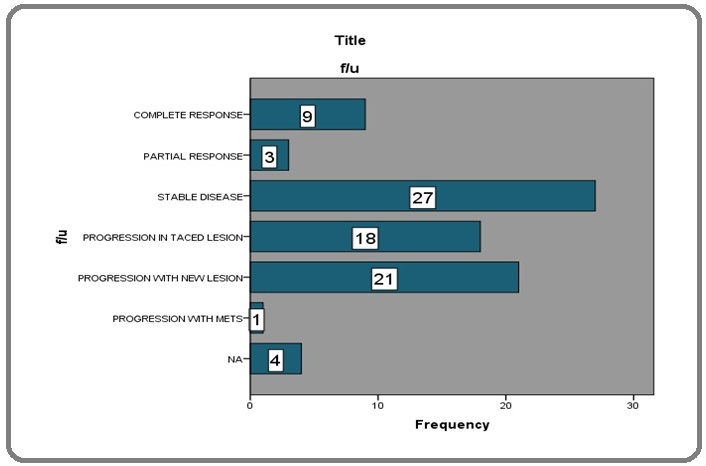
Tumor response
Tumor response was classified under the headings of completed response, partial response, stable disease, progression in lesion subjected to TACE, progression with development of new lesion and progression with metastatic disease (Figure 3).
Figure 3: Response Assessment of TACE.
Complications
Complications were classified under the headings of chemo related side effects, hepato-renal syndrome, Liver failure and death (Figure 4).
Figure 4:Complication in Percentages.
Survival rate
Our study indicated 1 year survival of 80% and 2 year survival of 56.6% post TACE (Figure 5).
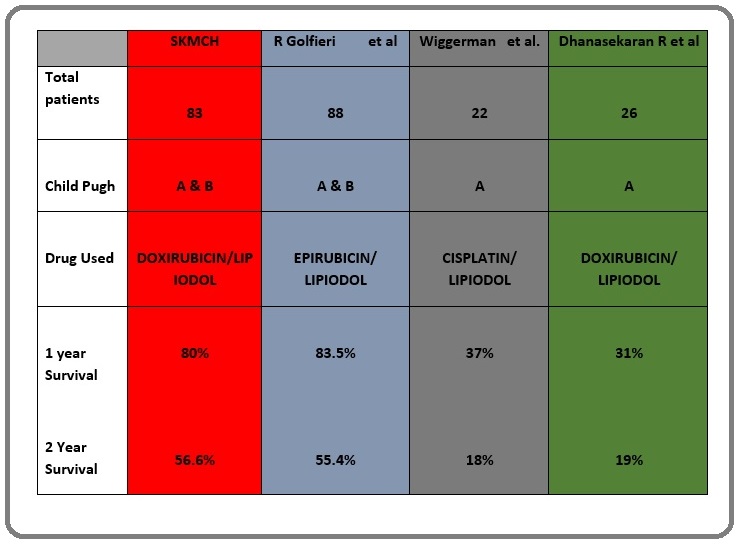
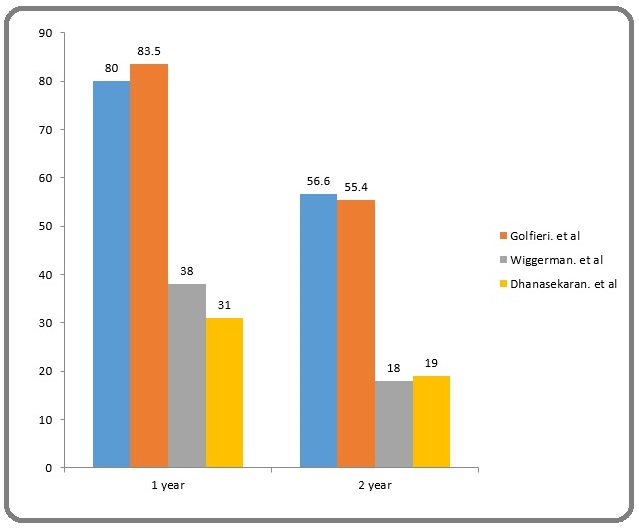
Figure 5: Comparative Analysis of Different Studies.
Discussion
Golfieri et al [2] published a study in 2014 comparing two year survival of conventional TACE (cTACE) versus DEB-TACE [2]. The estimated two year survival of cTACE was 55.4 % as compared to 56.6% of our study. Their study population included 88 patients. 13.5 % patients were Child’s B as compared to our 6.02 %. Additionally, the chemotherapeutic agent used was epirubicin. Another study conducted by Dhanasekaran R et al [10] from January 1998 up to July 2008 studying the comparing the outcome of cTACE with DEB-TACE showed a two year survival of 19 % for cTACE [10]. 26 out of the total of 71 of their patients underwent cTACE. 100% of their patient undergoing cTACE were child’s A. Cisplatin was the chemotherapeutic agent used in their study population. Wiggerman et al. [11] conducted a study in patients undergoing TACE from 2003-2008 which included 74 patients out of which 22 underwent cTACE [11]. All the 22 patients were child’s A, receiving cisplatin as chemotherapeutic agent. The estimated 2 year survival rate was 18 % (Figure 6).
Figure 6: 1 and 2 Year Survival Rates of Different Studies .
Acknowledgements
Limitations
1. 21 patients were excluded from the total population who lost to follow up.
2. Comparative studies compared cTACE with the DEB-TACE while our studies included the patients undergoing cTACE.
3. Child’s score
4. Drug used
References
- Patterns of Cancer Incidence, Mortality, and Prevalence Across Five Continents: Defining Priorities to Reduce Cancer Disparities in Different Geographic Regions of the World Kamangar Farin, Dores Graça M., Anderson William F.. Journal of Clinical Oncology.2006;24(14). CrossRef
- Randomised controlled trial of doxorubicin-eluting beads vs conventional chemoembolisation for hepatocellular carcinoma Golfieri R, Giampalma E, Renzulli M, Cioni R, Bargellini I, Bartolozzi C, Breatta A D, Gandini G, Nani R, Gasparini D, Cucchetti A, Bolondi L, Trevisani F. British Journal of Cancer.2014;111(2). CrossRef
- Universal Hepatitis B Vaccination in Taiwan and the Incidence of Hepatocellular Carcinoma in Children Chang Mei-Hwei, Chen Chien-Jen, Lai Mei-Shu, Hsu Hsu-Mei, Wu Tzee-Chung, Kong Man-Shan, Liang Der-Cherng, Shau Wen-Yi, Chen Ding-Shinn. New England Journal of Medicine.1997;336(26). CrossRef
- New frontiers in biomarkers for hepatocellular carcinoma Giannelli G., Antonaci S.. Digestive and Liver Disease.2006;38(11). CrossRef
- Seeding following percutaneous diagnostic and therapeutic approaches for hepatocellular carcinoma. What is the risk and the outcome? Stigliano R., Marelli L., Yu D., Davies N., Patch D., Burroughs A.K.. Cancer Treatment Reviews.2007;33(5). CrossRef
- Superselective transarterial chemoembolization for hepatocellular carcinoma. Validation of treatment algorithm proposed by Japanese guidelines Takayasu Kenichi, Arii Shigeki, Kudo Masatoshi, Ichida Takafumi, Matsui Osamu, Izumi Namiki, Matsuyama Yutaka, Sakamoto Michiie, Nakashima Osamu, Ku Yonson, Kokudo Norihiro, Makuuchi Masatoshi. Journal of Hepatology.2012;56(4). CrossRef
- Chemoembolization of hepatocellular carcinoma with drug eluting beads: Efficacy and doxorubicin pharmacokinetics Varela María, Real María Isabel, Burrel Marta, Forner Alejandro, Sala Margarita, Brunet Mercé, Ayuso Carmen, Castells Lluis, Montañá Xavier, Llovet Josep M., Bruix Jordi. Journal of Hepatology.2007;46(3). CrossRef
- The management of hepatocellular carcinoma. Current expert opinion and recommendations derived from the 10th World Congress on Gastrointestinal Cancer, Barcelona, 2008 Verslype C., Van Cutsem E., Dicato M., Arber N., Berlin J.D., Cunningham D., De Gramont A., Diaz-Rubio E., Ducreux M., Gruenberger T., Haller D., Haustermans K., Hoff P., Kerr D., Labianca R., Moore M., Nordlinger B., Ohtsu A., Rougier P., Scheithauer W., Schmoll H.-J., Sobrero A., Tabernero J., van de Velde C.. Annals of Oncology.2009;20. CrossRef
- Arterial embolisation or chemoembolisation versus symptomatic treatment in patients with unresectable hepatocellular carcinoma: a randomised controlled trial Llovet Josep M, Real Maria Isabel, Montaña Xavier, Planas Ramon, Coll Susana, Aponte John, Ayuso Carmen, Sala Margarita, Muchart Jordi, Solà Ricard, Rodés Joan, Bruix Jordi. The Lancet.2002;359(9319). CrossRef
- Comparison of conventional transarterial chemoembolization (TACE) and chemoembolization with doxorubicin drug eluting beads (DEB) for unresectable hepatocelluar carcinoma (HCC) Dhanasekaran Renumathy, Kooby David A., Staley Charles A., Kauh John S., Khanna Vinit, Kim Hyun S.. Journal of Surgical Oncology.2010. CrossRef
- Transarterial Chemoembolization of Child-A hepatocellular carcinoma: Drug-eluting bead TACE (DEB TACE) vs. TACE with Cisplatin/Lipiodol (cTACE) Wiggermann Philipp, Sieron Dominik, Brosche Christiane, Brauer Thomas, Scheer Fabian, Platzek Ivan, Wawrzynek Wojciech, Stroszczynski Christian. Medical Science Monitor.2011;17(4). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2020
Author Details