Prognostic Significance of Microvessel Density as Assessed by anti CD34 Monoclonal Antibody in Invasive Ductal Carcinoma of Breast
Download
Abstract
Context and Aim: Breast carcinoma is one of the leading causes of cancer related mortality in females. A number of established prognostic indicators exist for breast cancer. One potential indicator of adverse prognosis in breast cancer is tumor induced angiogenesis. Hematogenous spread of tumor cells is quantitatively related to microvessel density (MVD). This study aims to find possible association of microvessel density with other recognized prognostic factor such as patient age, tumor size, lymph node status, histologic grade, Nottingham Prognostic Index (NPI), vascular invasion, hormone receptor status and HER2/Neu expression in breast carcinoma.
Methods and Materials: This is a cross sectional, laboratory based descriptive type of observational study. This study was carried out in the Department of Pathology, SMS medical college. In our study we did histological evaluation of mastectomy specimen of breast cancer including ER, PR and Her 2 status and calculated the microvessel density (MVD) in appropriate section by applying IHC. MVD correlation with other clinicopathological parameters was done. Stastical analysis used in this study include Fischer Exact test and Chi Square test.
Results: A significant correlation was obtained between MVD and tumor size, lymph node metastasis, lymphovascular invasion, histologic grade and NPI (P value of 0.01).
Conclusions: Angiogenesis is an important component of cancer growth, invasion and metastasis. Therefore, inhibition of angiogenesis is an attractive strategy for treatment of cancer. The correlation of MVD with prognosis suggests the utility of antiangiogenic drugs in breast cancer patients with high angiogenesis
Introduction
Breast carcinoma is one of the leading causes of cancer related mortality in females. It is the most common cancer among women in India with an incidence of approximately 1,45,000 cases annually and around 70,000 deaths annually [1].
In developing countries peak incidence of breast carcinoma occurs in 45 to 60 years. Despite various screening modalities, diagnosis and treatment employed, many women die from disease each year. A number of established prognostic indicators exist of breast cancer, which includes tumor size, lymph node status, histological grade, tumor type, vascular invasion and hormone receptor status. Of these, axillary lymph node status has been regarded as the most important prognostic marker [2].
However 20-30% of all lymph node negative patients will still develop a recurrence of the disease within 10 years of initial treatment of primary tumor.
Therefore it is clear that a new prognostic marker that could identify the patients at high risk of tumor recurrence more accurately would be of great value and would allow more appropriate and effective treatment of those at greatest risk. One potential indicator in breast cancer is tumor induced angiogenesis. Angiogenesis is a prerequisite for tumor growth and metastasis.
Neovascularization provides not only the route for nutrient supply to the tumor but also the conduit for tumor cells to be shed in the circulation.
It has been demonstrated that the increasing density of newly formed microvessels in growing tumor correlated closely with the increasing number of tumor cells shed into the blood stream. Thus signifying that the hematogenous spread of tumor cells is quantitatively related to microvessel density (MVD). This study aims to determine microvessel density in invasive ductal carcinoma of breast and its clinicopathological correlation with other prognostic factors such as patient age, tumor size , lymph node status,type of tumor, grade, vascular invasion, estrogen progesterone receptor status, tumor epidermal growth factor (HER2/Neu) expression in breast carcinoma.
Materials and Methods
In our study 65 specimens diagnosed as invasive duct carcinoma of breast by histopathology were included; we did histological evaluation of mastectomy specimen of breast cancer, determined the histological grade and the Nottingham prognostic Index (NPI Score) and the ER, PR and Her 2 status of each case. We used Anti CD34 monoclonal antibody (clone QBEND/10 prediluted, RTU) by immunohistochemical staining to calculate microvessel density in appropriate section.
Assessment of MVD
MVD was evaluated by counting anti-CD34 positive microvessels and calculated by the counting method developed by Weidner et al [3] using a light microscope.
Accordingly, after scanning the whole tumor section with a light microscope under a low magnification (100X), the area with highest number of microvessels was identified as ‘hot-spot’ (Figure 1) and microvessels were counted under a higher magnification (400X) in this area.
Figure 1: Angiogenesis in Invasive Breast Carcinoma. Identifying the hot spots of microvessel density (CD34 positive endothelial cells) at100x.
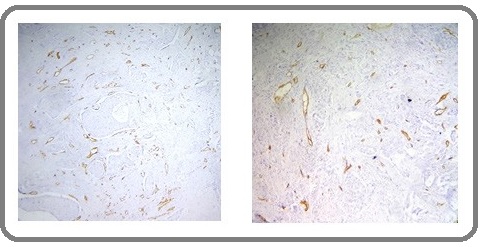
Any brown-stained single endothelial cell or endothelial cell clusters separated from surrounding tumor cells and connective tissue elements were considered to be a microvessel regardless of whether they had a lumen or not. No erythrocyte was necessarily required in the lumen. Branching vessel structures were counted as a single vessel. Vessels of a caliber larger than approximately eight red blood cells, vessels with thick muscular walls, and vessels in sclerotic areas were excluded from the count. Vascularity was not considered in the areas of necrosis within the tumor. After determining microvessel counts of all patients, the mean MVD was determined. This value was regarded as the cut-off value. Patients with microvessel count below this cut-off value were classified as ‘low MVD’ and patients with microvessel count above this cut-off value were classified as ‘high MVD’.
Data entry and statistical analysis
The collected data was transformed into variables,coded and entered in Microsoft Excel. Data were analyzed and statistically evaluated using SPSS-PC-17 version. Quantitative data was expressed in mean and standard deviation while qualitative data was expressed in percentage. Statistical differences between the proportions were tested by Chi square test or Fisher’s exact test. ‘P’ value less than 0.05 was considered statistically significant.
Results
We conducted this study on 65 patients of breast carcinoma. The patients were women in the age range of 20 to 87 years. Majority of the cases were in the age group of 45-54 years (26.2%) followed by the age group 35 to 44 years (24.6%). Lowest number of cases 2 (3.1%) was found to be in the age group of <25 years. The tumor size varied from 1.2 to 11 cm in largest dimension. Lymph node metastasis was present in 40 (61.5%) cases. Lymphovascular invasion was present in 39 (60%) cases while it was absent in 26 (40%) cases. NPI of our patients ranged from 2.2 to 8.2 with a mean standard deviation of 4.728 ± 1.4626.
The Microvessel density (MVD) was measured in all 65 patients and ranged from 6/ HPF to 24/HPF with a mean standard deviation of 14.78 ± 4.768. The mean MVD of 14.78 was taken as the cut off value and cases were classified in two groups: those with MVD score < 14.78 as low MVD (n= 33 cases) and ≥ 14.78 as high MVD (n=32 cases).
MVD correlation with T stage, lymph node status, lymphovascular invasion, histologic grade and NPI: There exists a significant positive correlation between higher T stage and higher MVD (Table 1) and between lymph node metastasis and high MVD (Table 2).
| T stage | Low MVD (%) | High MVD (%) | Total |
| T1 | 8 (100.0%) | 0 (0.0%) | 8 |
| T2 | 24 (48.0%) | 26 (52.0%) | 50 |
| T3/T4 | 1 (14.3%) | 6 (85.7%) | 7 |
| Total | 33 | 32 | 65 |
| Nodal Status | Low MVD (%) | High MVD (%) | Total |
| Negative | 22 (88.0%) | 3 (12.0%) | 25 |
| Positive | 11 (27.5%) | 29 (72.5%) | 40 |
| Total | 33 | 32 | 65 |
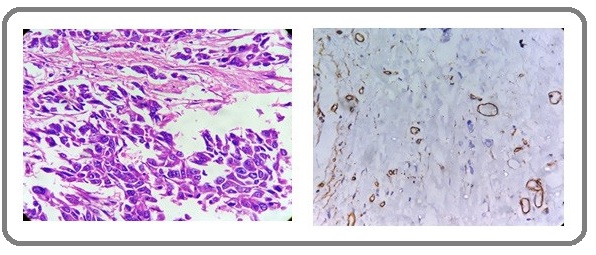
In histologic grade 1, all the 15 (100%) cases had low MVD. In grade 2, there were 15 (57.7 %) cases with low MVD and 11 (42.3%) cases with high MVD. In grade 3 majority of the cases 21 (87.5%) had high MVD. There was a significant positive correlation between high histologic grade and high MVD (Table 3 and Figure 2,3 and 4).
| Histological grade | Low MVD (%) | High MVD (%) | Total |
| 1 | 15 (100.0%) | 0 (0.0%) | 15 |
| 2 | 15 (57.7%) | 11 (42.3%) | 26 |
| 3 | 3 (12.5%) | 21 (87.5%) | 24 |
| Total | 33 | 32 | 65 |
Figure 2: H &E Section and IHC Section Showing Low MVD in Grade 1 Invasive Ductal Carcinoma (400x).
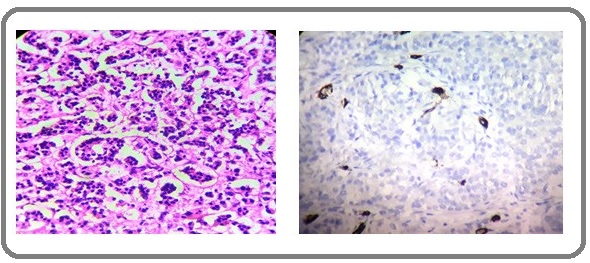
Figure 3: H & E Section and IHC Section Showing MVD in Grade 2 Invasive Ductal Carcinoma (400x).
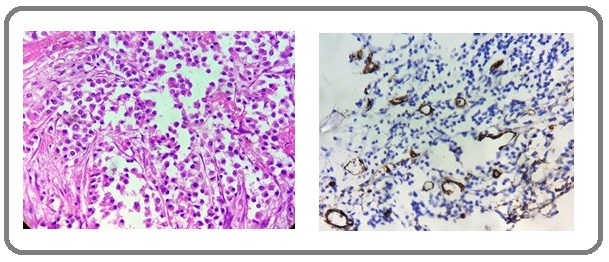
Figure 4: H & E Section and IHC Section Showing High MVD in Grade 3 Invasive Ductal Carcinoma (400x).
Lymphovascular invasion was present in 39 (60%) cases. There was a significant positive correlation between presence of lymphovascular invasion and high MVD (Table 4).
| Lymphovascular invasion | Low MVD (%) | High MVD (%) | Total |
| Present | 12 (30.8%) | 27 (69.2%) | 39 |
| Absent | 21 (80.8%) | 5 (19.2%) | 26 |
| Total | 33 | 32 | 65 |
All the 17 (100%) cases in NPI group A had low MVD. In NPI group B, 12 (57.1%) cases had low MVD and 9 (42.9%) cases had high MVD. In NPI group C only 4 (14.8%) cases had low MVD and majority of the cases 23 (85.2%) had high MVD. There was a significant positive correlation between increasing NPI and high MVD (Table 5).
| NPI | Low MVD (%) | High MVD (%) | Total |
| Group A | 17 (100.0%) | 0 (0.0%) | 17 |
| Group B | 12 (57.1%) | 9 (42.9%) | 21 |
| Group C | 4 (14.8%) | 23 (85.2%) | 27 |
| Total | 33 | 32 | 65 |
There was no significant correlation between MVD and ER, PR and HER 2/Neu status.
Discussion
In this study, we have determined angiogenesis in invasive ductal carcinoma by counting microvessels using anti CD34 antibody and compared MVD that we obtained from each patient with other prognostic factors. We followed the method of counting vessels as described by Weidner et al [3] by finding the vascular hotspots at low power and point count at 400x magnification. In this study we tried to find any association of microvessel density with prognostic factors as tumor size, axillary lymph node status, histologic grade, Nottingham prognostic index, lymphovascular invasion, hormone receptor and Her-2-neu status. The mean MVD of 14.78/ HPF with a standard deviation of 4.768 (average range, 6 to 24) was obtained in our study. Pyakurel et al [2] in their study calculated a range of microvessel density of 3.8 to 29.3 with a mean of 17.97 (vv/mm2) and a standard deviation of 7.147. The mean and median microvessel counts from the study of Weidner et al [3] was 60 and 56 (range, 8 to 167), respectively, with a 0.74 mm2 counting area.
In the present study we have found a statistically significant correlation between increasing tumor size and high MVD with a P value of 0.01. Similar findings were observed in the studies done by Horak et al, [4] Weidner et al [3] and Sener et al [5]. However, in the study done by Pyakurel et al [2] no significant correlation was found between these variables with a P value of 0.065, this could be due to the low sample size of the study (35 patients). Studies with a larger cohort are thus needed to establish a relationship between two variables.
The MVD and lymph nodes metastasis was correlated in this study with a significant P value ≤0.01. These findings were in concordance with the studies done by Horak et al, [4] Weidner et al, [3] Bosari et al [6] and Sener et al [5] who found a significant relationship between increased MVD and metastasis to lymph nodes.
In our study a significant positive correlation was found between the MVD and histologic grade with a P value of ≤ 0.01. Our findings are in accordance with the findings of studies done by Pyakurel et al, [2] Safwat et al, [7] Bolat et al, [8] Kwon et al. [9]. In study of Pyakurel et al, [2] the microvascular density and histologic grade showed significant relation with a P value of 0.006. (Table 6).
| Study | Tumor size | Lymph node metastasis | Tumor grade | Lymphovascular invasion | NPI |
| (P value) | (P value) | (P value ) | (P value) | (P value) | |
| Pyakurel et al [2] | 0.065 | 0.074 | 0.006 | 0.755 | 0.003 |
| Kwatra et al [10] | 0.007 | 0.001 | 0.05 | <0.05 | 0.01 |
| Sener et al [5] | 0.001 | 0.05 | >0.05 | 0.169 | >0.05 |
| Bosari et al [6] | Not significant | <0.001 | Not significant | 0.002 | Not done |
| Dhakal et al [15] | <0.001 | 0.28 | <0.001 | 0.006 | Not done |
| Present study | 0.01 | ≤0.01 | ≤0.01 | <0.01 | <0.01 |
Our study also showed significant positive correlation of MVD with two other important prognostic factors that are lymphovascular invasion and NPI with a P value of <0.01 in both. These findings were also in concordance with the studies done by Kwatra et al [10] and Pyakurel et al [2]. No significant relationship was found between MVD and other clinicopathologic parameters such as patient’s age, estrogen- progesterone receptor status, Her-2-neu overexpression and triple negative cases in the present study. In the literature, there are studies by Wang et al [11] and Erdem et al [12] that have reported no significant relation between MVD and clinicopathologic parameters mentioned above.
Low microvessel count seems to be an excellent marker to identify patients with good prognosis. Contradictions and inconsistencies have been demonstrated in the studies carried out, although the majority found that high MVD correlated with poor prognosis, higher histological grade and lymph node metastasis. The reasons for these variations include the different investigation methods employed, technical problems (e.g. variability in immune staining) and the subjectivity involved in the process of selection and counting.If these results are confirmed in larger studies, it might be possible to add microvessel count to other prognostic factors to identify patients at high risk for recurrence and to guide decisions on adjuvant systemic therapy.There is a strong rationale for the usage of antiangiogenic therapies in early, locally advanced and metastatic breast cancer. The concentration of hypoxia-inducible factor (HIF-1) alpha, a key player in angiogenesis regulation, is higher in breast tumors than in normal breast tissue and is even higher in poorly differentiated lesions than in the corresponding type of well-differentiated lesions [13].There are a number of inhibitors of angiogenesis currently used in clinical practice targeting different factors. Breast cancer is paramount in terms of specific targets for cancer therapy. The antibodies combination targeting both the HER family and angiogenic pathways (e.g., trastuzumab plus bevacizumab) is valuable for clinical setting. The use of these therapies requires robust evidence for cancer reduction and metastasis prevention without major drug toxicities.In conclusion, the results of our study thus suggest an association of high MVD with factors associated with poor prognosis in breast cancer, such as larger tumor size, lymph node metastasis, high histologic grade, lymphovascular invasion and higher Nottingham prognostic index. We could not however show a significant correlation with other known variables like ER, PR and Her-2-neu status, probably because of small sample size.
In our study high MVD is found to be a significant unfavorable prognostic factor in breast cancer. Angiogenesis is an important component of cancer growth, invasion and metastasis. It could be a likely therapeutic target for antiangiogenic therapy. Therefore, inhibition of angiogenesis is an attractive strategy for treatment of cancer [14].
Additional work on anti-angiogenic factors needs to be done so as to elucidate better targeted therapies which will have profound effect on reducing tumor burden and prevent metastasis without major drug toxicity.
References
- Estimating the world cancer burden: Globocan 2000 Parkin D. Maxwell, Bray Freddie, Ferlay Jacques, Pisani Paola. International Journal of Cancer.2001;94(2). CrossRef
- A study on microvascular density in breast carcinoma Pyakurel D, Karki S, Agrawal CS. J Pathol Nepal.2014;4:570-575.
- Tumor Angiogenesis and Metastasis — Correlation in Invasive Breast Carcinoma Weidner Noel, Semple Joseph P., Welch William R., Folkman Judah. New England Journal of Medicine.1991;324(1). CrossRef
- Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer Horak E.R., Klenk N., Leek R., LeJeune S., Smith K., Stuart N., Harris A.L., Greenall M., Stepniewska K.. The Lancet.1992;340(8828). CrossRef
- Comparison of microvessel density with prognostic factors in invasive ductal carcinomas of the breast Sener Ebru, Sipal Sare, Gundogdu Cemal. Turkish Journal of Pathology.2016. CrossRef
- Microvessel quantitation and prognosis in invasive breast carcinoma Bosari Silvano, Lee Arthur K.C., DeLellis Ronald A., Wiley Brian D., Heatley Gerald J., Silverman Mark L.. Human Pathology.1992;23(7). CrossRef
- Morphometric and immunohistochemical study of angiogenic marker expressions in invasive ductal carcinomas of the human breast Safwat MD, Habib F, Elayat A, Oweiss N, Reffat S, Algaidi S. Folia Morphol.2009;68(3):144-155.
- Microvessel density, VEGF expression, and tumor associated macrophages in breast tumors: Correlations with prognostic parameters Bolat F, Kayaselcuk F, Nursal TZ, Yagmurdur MC, Bal N, Demirhan B. J Exp Clin Cancer Res .2006;25:365-372.
- Mast Cell and Macrophage Counts and Microvessel Density in Invasive Breast Carcinoma-Comparison Analysis with Clinicopathological Parameters Kwon Gui Young, Lee Sang Dae, Park Eon Sub. Cancer Research and Treatment.2005;37(2). CrossRef
- Correlation of various histopathologic prognostic factors with Nottingham prognostic index and microvessel density in invasive breast carcinoma: A study of 100 cases Singh S, Kwatra A, Aggarwal D, Gupta R, Chaturvedi AK, Kudesia M. Indian Journal of Cancer.2015;52(1). CrossRef
- Microvessel density recognized by Endoglin as prognostic markers in breast carcinoma Wang G, Liang Y, Zhang H, Wang L, Xu J. J Mater Sci.2014;3:41-46.
- The prognostic value of p53 and c-erbB-2 expression, proliferative activity and angiogenesis in node-negative breast carcinoma Erdem O, Dursun A, Coskun U, Gunel N. Tumori.2005;91(1):46-52.
- Levels of Hypoxia-Inducible Factor-1 During Breast Carcinogenesis Bos R., Zhong H., Hanrahan C. F., Mommers E. C. M., Semenza G. L., Pinedo H. M., Abeloff M. D., Simons J. W., van Diest P. J., van der Wall E.. JNCI Journal of the National Cancer Institute.2001;93(4). CrossRef
- Antiangiogenic therapy for breast cancer Nielsen Dorte Lisbet, Andersson Michael, Andersen Jon Lykkegaard, Kamby Claus. Breast Cancer Research.2010;12(5). CrossRef
- Vascularization in Primary Breast Carcinomas: Its Prognostic Significance and Relationship with Tumor Cell Dissemination Dhakal H. P., Naume B., Synnestvedt M., Borgen E., Kaaresen R., Schlichting E., Wiedswang G., Bassarova A., Giercksky K.-E., Nesland J. M.. Clinical Cancer Research.2008;14(8). CrossRef
License
Copyright
© ,
Author Details