Study of Serum CEA and Ca 19.9 in Esophageal Squamous Carcinoma and ROC Curve Analysis
Download
Abstract
Background: There are very few studies on the comparative diagnostic value of CEA and Ca19.9 in esophageal squamous cell carcinoma (ESCC).
Aims and objectives: The aim of the present study was to access the clinical relevance of CEA and CA19.9 in ESCC and to determine whether combined use of these marker could improve diagnostic sensitivity and specificity.
Material and Method: Venous blood Samples were collected from total 68 individuals, divided into two categories, group 1 includes 18 healthy individuals and group 2 includes 50 patients with already diagnosed cases of ESCC prior to any treatment. Tests were performed to estimate the value of CEA and Ca19.9. Different statistical analysis has been performed to derive a clinically meaningful value.
Results: The mean serum CEA and CA 19.9 levels and also the ratio of CEA/Ca19.9 were higher in patients with ESCC than healthy subjects. In ESCC, the optimal combination of sensitivity and specificity for CEA were determined as 48% and 94% and for CA19.9 were determined as 76% and 72% respectively, whereas combined analysis of CEA/CA19.9 were lower than individual value in patients. Over all accuracy rate was better with CA19. The diagnostic power of CEA and CA19-9 as a screening test for ESCC were assessed by ROC curve analysis. The cut-off value for CEA and CA19-9 in ESCC were found to be 2.9 2 ng/ml and 7.4 ng/ml and were found to be above the cut-off value in 25 (50%) and 42 (84%) of the patients with ESCC respectively.
Conclusion: The results of our study indicated that serum CA19.9 has a higher positivity than serum CEA in esophageal cancer. The combined use of CA19-9 and CEA (ratio) could not increase the diagnostic sensitivity in esophageal cancers in our study.
Introduction
Esophageal cancer (EC) is the eighth-most common cancer and the sixth-most common deadliest cancer worldwide [1]. In India, it is the fourth most common cause of cancer-related deaths [2], whereas in North-east Indian population esophageal cancers are the most common malignancy in male, second most common cancer in female and second most in overall population as per kamrup urban registry [3]. Much regional variation exists in the incidence and pathology of esophageal cancer. Although majority of cancer are squamous cell carcinoma (SCC), it has been reported that in countries with higher human development index (HDI), there is a higher incidence of adenocarcinoma (AC) of the esophagus [4]. Currently, in Indian subcontinent, the most common type of EC is SCC the and the most common location is the distal third of the esophagus [5]. Approximately, 47,000 new cases are reported each year and the reported deaths reach up to 42,000 each year in India [6]. Major risk factors for SCC are predicted to be poor nutritional status, low intake of fruits and vegetables, drinking beverages at high temperatures excess tobacco and alcohol consumption, and possibly human papillomavirus infection., whereas the established risk factors for AC esophagus include smoking, alcohol, obesity, chronic gastroesophageal reflux disease, and the presence of Barrett’s esophagus.
Here, it is worth mentioning that published data from different regions in India have indicated their observations on the local risk factors. For example, the northern state of Kashmir, smoking (hookahs), snuff, sundried spices and vegetables, hot salted tea with baking soda, and red chilies have been implicated as risk factors [2-7], whereas in North-east India Betel quid chewing with or without tobacco consumption is associated with the development of EC specially in Assam [8]. Kalakhar, a unique and locally made food item, has emerged as a significant risk factor (odds ratio [OR] =8.0, 95% confidence interval [CI] =5.1–11.5, P < 0.001) associated with esophageal cancer [9].
Another publication from Ludhiana, Punjab, found that poor nourishment and consumption of hot beverages linked to SCC when they studied esophageal SCC in women who generally neither smoke nor consume alcohol [10].
The cause of deadliness of these tumour are due to late stage presentation, when advanced SCC form mass and invades lymph nodes and also due to subtle early lesions easily missed on endoscopy in asymptomatic patients. The another attributable cause may be lack of dedicated tumour marker for EC screening. In other gastrointestinal (GI) cancers, routinely used markers like serum CEA and CA 19.9 are confined to more of prognostic and treatment response parameter than diagnostic or screening tool. As the individual value of these marker are influenced by benign and inflammatory processes, the combination of these serum markers might be of useful in diagnostic assessment.
There are very few studies on the comparative diagnostic value of CEA and Ca19.9 in esophageal squamous cell carcinoma (ESCC). From India, Bagaria et al has studied comparative diagnostic value of CEA and Ca19.9 in esophageal , gastric and colon cancer [11]. The aim of the present study was to access the clinical relevance of CEA and CA19.9 in ESCC and to determine whether combined use of these marker could improve diagnostic sensitivity and specificity by analysing ROC curve.
Materials and Methods
After analysing the availability of all the inclusion data and exclusion criteria, the study subjects include 68 individuals, divided into two categories, group 1 includes 18 healthy individuals and group 2 includes 50 patients with already diagnosed cases of esophageal squamous cell carcinoma prior to any treatment. Exclusion criteria used for healthy group were use of tobacco any form, any GI illness or recent hospitalization, considering these factors might influence the serum tumour markers levels. Cancer patients with history of any form of treatment were excluded from the study.
Venous blood Samples were collected from each subject in clot vials. Blood was then centrifuged at 2000 rpm for 10 min in a refrigerated centrifuge to separate serum samples from the cells. CEA, CA19-9 levels were measured using the commercial IMMULITE CEA and CA19-9 kits, which were a solid-phase, two site chemiluminescent immunometric assay.
Statistical analysis
All results were given as (mean±SD). The difference of serum levels of CEA and CA19-9 between group 1 and group 2 were compared by one way- ANOVA model using Scheffe test. The difference was considered statistically significant if the value was less than 0.05.
Sensitivity, specificity, positive and negative predictive value, positive and negative likelihood ratio, accuracy rate were calculated by using the standard methods.
A receiver operating characteristic (ROC) curve was constructed to decide the cut-off point and to assess the diagnostic accuracy of each tumor marker value individually as well as ratio for the diagnosis of ESCC.
Results
Data were analyzed using SPSS version 10.0. The mean age of the patients were 50 year with age range from 26-70 year and male to female ratio of 3:1. No significant difference in age and gender were found between the healthy and patient group.
The mean serum levels of the individual tumor markers were shown in Table 1.
| Descriptives | |||||
| N | Mean | Std. Deviation | p value | ||
| cea | Group1 (Control) | 18 | 1.858 | 0.744 | |
| Group 2 (Esophageal Cancer) | 50 | 11.261 | 56.148 | 0.482 | |
| ca19.9 | Group1 (Control) | 18 | 8.8 | 8.164 | |
| Group 2 (Esophageal Cancer) | 50 | 44.316 | 143.072 | 0.299 |
The mean serum CEA and CA 19.9 levels in patients with ESCC were higher than healthy subjects. But there were no significant differences in serum CEA and CA 19. 9 levels between the two groups (p>0.05).
The mean of ratio CEA/CA19.9 is more in group 2 than that of group 1, however statistical significance is not seen Table 2.
| Ratio | Study group | N | Mean | Std deviation | pvalue |
| CEA/Ca 19.9 | Group 1 | 18 | 0.439 | 0.489 | 0.958 |
| Group 2 | 50 | 0.449 | 0.756 |
The sensitivity, specificity, negative and positive predictive value and likelihood ratio of CEA and CA19.9 are mentioned in the Table 3,4 and 5. In ESCC, the optimal combination of sensitivity and specificity for CEA were determined as 48% and 94% respectively, and for CA19-9 sensitivity and specificity were determined as 76% and 72% respectively.
| Statistic | Value | 95% Cl |
| Sensitivity | 48.00% | 33.66% to 62.58% |
| Specificity | 94.44% | 72.71% to 99.86% |
| Positive Likelihood Ratio | 8.64 | 1.26 to 59.32 |
| Negative Likelihood Ratio | 0.55 | 0.41 to 0.74 |
| Disease Prevalence (*) | 73.53% | 61.43% to 83.50% |
| Positive Predictive Value (*) | 96.00% | 77.76% to 99.40% |
| Negative Predictive Value (*) | 39.53% | 32.88% to 46.61% |
| Accuracy (*) | 60.29% | 47.70% to 71.97% |
| Statistic | Value | 95% Cl |
| Sensitivity | 76.00% | 61.83% to 86.94% |
| Specificity | 72.22% | 46.52% to 90.31% |
| Positive Likelihood Ratio | 2.74 | 1.28 to 5.86 |
| Negative Likelihood Ratio | 0.33 | 0.19 to 0.59 |
| Disease Prevalence (*) | 73.53% | 61.43% to 83.50% |
| Positive Predictive Value (*) | 88.37% | 78.02% to 94.21% |
| Negative Predictive Value (*) | 52.00% | 37.98% to 65.71% |
| Accuracy (*) | 75.00% | 63.02% to 84.71% |
| Statistic | Value | 95% Cl |
| Sensitivity | 52.00% | 37.42% to 66.34% |
| Specificity | 50.00% | 26.02% to73.98% |
| Positive Likelihood Ratio | 1.04 | 0.61 to 1.77 |
| Negative Likelihood Ratio | 0.96 | 0.56 to 1.66 |
| Disease Prevalence (*) | 73.53% | 61.43% to 83.50% |
| Positive Predictive Value (*) | 74.29% | 62.89% to 83.12% |
| Negative Predictive Value (*) | 27.27% | 17.87% to 39.27% |
| Accuracy (*) | 51.47% | 39.03% to 63.78% |
Combined analysis of CEA/CA19.9 showed that sensitivity and specificity were lower than individual value in patients with esophageal cancer. Over all accuracy rate was better with CA19.9.
The diagnostic power of serum CEA and CA19-9 as a screening test for esophageal squamous cell carcinoma were assessed by ROC curve analysis.
In a receiver operating characteristic (ROC) curve, the true positive rate (sensitivity) is plotted as a function of the false positive rate (100% specificity) for different threshold values. Each point on the ROC curve represents a sensitivity/specificity pair corresponding to a particular decision threshold. A test with perfect discrimination (no overlap in the two distributions) has a ROC curve passing through the upper left corner (100% sensitivity, 100% specificity). Therefore, a ROC curve that is closer to the upper left corner indicates a higher overall accuracy of the test.
Using ROC curve (Figure 1), the cut-off value for serum CEA and CA19-9 in esophageal squamous cell Carcinoma were found to be 2.9 2 ng/ml and 7.4 ng/ml.
Serum CEA values were found to be above the cut-off value of 2.92 ng/mL in 25 (50%) of the patients with esophageal squamous cell carcinoma, whereas Serum CA 19-9 values were found to be above the cut-off value of 16.5 U/mL, in 42 (84%) of the patients with esophageal squamous cell carcinoma.
Discussion
Esophageal cancer is one of the commonest malignancy in our area and since there is no ideal serum markers for gastrointestinal malignancy other than CEA and CA19.9, we attempted to find a meaningful correlation with these serum markers in esophageal cancers mainly squamous cell carcinoma. There are very limited number of studies of serum markers in ESCC in the literature. There is one study by Bagaria et al [11]., found that combination of CEA and CA19-9 exhibits higher diagnostic efficiency than individual tumor marker in esophageal and gastric cancer.
The present study was done to determine clinical value of individual and combined use of serum CEA and CA 19.9 in esophageal squamous cell carcinoma.
CEA is a glycosylphosphatidylinositol-cell surface anchored glycoprotein is currently classified under the immunoglobulin super family and functions as an intracellular adhesion molecule. Originally described by Gold and Freedman in 1965, CEA has specialized sialofucosylated glycoforms which act as functional colon carcinoma L-selectin and E-selectin ligands, thereby significantly affecting the metastatic dissemination of colon carcinoma [12-13].
The CA19.9 antigen was first isolated by Koprowski et al. in 1979 is a type of glycosphingolipid that is a specific sialyzed derivative of the Lea blood group and shown as Lexa [14].
The significance of serum CEA levels is well established in colon cancer detection, determination of stage and recurrence, and evaluation of cancer therapy [15]. But the role of CEA in patients with esophageal cancer and gastric cancer is still controversial [16]. A few studies have mentioned the beneficial role of CEA in determining the relapses and the follow up of the responses to the treatment of the patients with gastric and esophageal cancers [17].
Other than gastrointestinal malignancies, CA 19.9 is found to be elevated in hepatico- pancreatic carcinoma, ovarian mucinous carcinoma [18] and CEA is found elevated in lung carcinoma, and breast carcinoma, as well as those with medullary thyroid carcinoma [19].
The advantage of combined use of multiple tumor markers is under debate for patients with gastrointestinal tumors in the literature.
Our study also revealed that the mean serum CEA and CA19.9 levels in patients with esophageal cancer were higher than healthy subjects, however no statistical significance was found between the two groups like in most of the studies.
A receiver operating characteristic (ROC) curve was analyzed to decide the cut-off point and to assess the diagnostic accuracy of each tumor marker value for the diagnosis of esophageal SCC.
In our study, using ROC curve (Figure 1), the cut-off value for serum CEA and CA19-9 in esophageal squamous cell Carcinoma were found to be 2.9 2 ng/ml and 7.4 ng/ ml. Our findings correlates with Tuncer et al. and Bargaria et al for CEA but contradictary to CA19.9 levels.
Figure 1: ROC Curve of CEA and CA19.9.
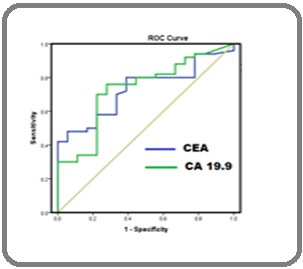
We have found Serum CEA values above the cut-off value of 2.92 ng/mL in 25 (50%) of the patients with esophageal squamous cell carcinoma, whereas Serum CA 19-9 values were found to be above the cut-off value of 16.5 U/mL, in 42 (84%) of the patients with esophageal squamous cell carcinoma. This corresponds to Tuncer et al [20] findings, whereas Bargaria et al [11] found CEA elevation in 38% and CA 19.9 in 18 % of esophageal SCC.
Gion et al [21] found that CA19-9 was positive in 13% of esophageal cancers and CEA was positive in 27% of the patients with esophageal cancers.
We have found CA19.9 has highest diagnostic accuracy than CEA and CEA/CA19.9 ratio in contrast to Bargeria et al [11] as they concluded combination serum markers exhibits higher diagnostic efficiency than individual tumor marker in esophageal SCC.
Tuncer et al [20] present study showed that CEA has a higher positivity rate for esophageal squamous cell carcinoma than CK-18 and CA19-9. The variation in the finding is not yet explainable, may be requiring more studies at genetic or epigenetic levels.
Our study revealed similar results as Patai et al [22] reported that the combined use of CA19-9 and CEA (ratio) could not increase the diagnostic sensitivity in gastrointestinal cancers.
The varying results of different studies offer no conclusions regarding diagnostic efficacy of routinely used serum markers. But the clinical use of tumor markers is much more beneficial in determination of prognosis, assessing response to treatment and detection of early recurrences [23].
CEA has been reported to be beneficial in determining the relapses and the follow up of the responses to the treatment of the patients with gastric and esophageal cancers [24].
Tokunaga et al17 found from their study that preoperative serum CA19-9 is a useful prognostic marker in patients with cardio-esophageal junction adenocarcinoma and also mentioned that serum CEA and CA 19.9 levels in all subjects with esophageal cancer is important for detection of possible liver metastasis and pancreatic invasion. According to Munck-Wikland et al [25], the appearance of distant metastases is associated with increased CEA levels in esophageal cancer.
In conclusion, the results of our study indicated that serum CA19.9 has a higher positivity than serum CEA in esophageal cancer. The combined use of CA19-9 and CEA (ratio) could not increase the diagnostic sensitivity in esophageal cancers in our study. Studies on the utilization of these markers as predicting prognosis rather than in early diagnosis should be considered.
Acknowledgements
Our technician Mr. Ratul Haloi for his support.
References
- Esophageal cancer: A Review of epidemiology, pathogenesis, staging workup and treatment modalities Napier Kyle J. World Journal of Gastrointestinal Oncology.2014;6(5). CrossRef
- Esophageal cancer in India: Current status and future perspectives Samarasam Inian. International Journal of Advanced Medical and Health Research.2017;4(1). CrossRef
- National cancer registry program three year report of population based cancer registries: 2012-2014: report of 27PBCRs in India Indian council of Medical Research..
- Global cancer transitions according to the Human Development Index (2008–2030): a population-based study Bray Freddie, Jemal Ahmedin, Grey Nathan, Ferlay Jacques, Forman David. The Lancet Oncology.2012;13(8). CrossRef
- Carcinoma of the esophagus in Tamil Nadu (South India): 16‑year trends from a tertiary center Cherian JV, Sivaraman R, Muthusamy AK, Jayanthi V. J Gastrointestin Liver Dis .2007;16:245-249.
- GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 11. Lyon, France: International Agency for Research on Cancer; 2013 Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, et al . Available from: http://www.globocan.iarc.fr. [Last accessed on 2017 Apr 24]..
- Risk factors and survival analysis of the esophageal cancer in the population of Jammu, India Dhar MK, Sehgal S, Kaul S, Gupta BB. Indian Journal of Cancer.2012;49(2). CrossRef
- Gene expression profile of esophageal cancer in North East India by cDNA microarray analysis Chattopadhyay Indranil. World Journal of Gastroenterology.2007;13(9). CrossRef
- Role of Dietary Habits in the Development of Esophageal Cancer in Assam, the North-Eastern Region of India Kumar Phukan Rup, Kanta Chetia Chandra, Shahadat Ali Mir, Mahanta Jagadish. Nutrition and Cancer.2001;39(2). CrossRef
- Risk factors analysis of squamous cell carcinoma (SCC) esophagus in North Indian females in tertiary care hospital: A case–control study Das KC, Singh S, Pawar G, Masih R, Raju N. Int J Recent Sci Res.2015;6:4661-4664.
- Comparative study of CEA and CA19-9 in esophageal, gastric and colon cancers individually and in combination (ROC curve analysis) Bagaria B, Sood S, Sharma R, Lalwani S. Cancer Biol Med .2013;10:148-157. CrossRef
- Carcinoembryonic Antigen and CD44 Variant Isoforms Cooperate to Mediate Colon Carcinoma Cell Adhesion to E- and L-selectin in Shear Flow Thomas Susan N., Zhu Fei, Schnaar Ronald L., Alves Christina S., Konstantopoulos Konstantinos. Journal of Biological Chemistry.2008;283(23). CrossRef
- Cancer Cells in Transit: The Vascular Interactions of Tumor Cells Konstantopoulos Konstantinos, Thomas Susan N.. Annual Review of Biomedical Engineering.2009;11(1). CrossRef
- Carbohydrate antigen 19-9 (CA 19-9) (serum, plasma) The Association for Clinical Biochemistry 2012..
- CA72-4 compared with CEA and CA19-9 as a marker of some gastrointestinal malignancies Lopez JB, Royan GP, Lakhwani MN, Mahadaven M, Timor J. Int J Biol Markers.1999;14:172-177.
- The prognostic value of preoperative serum levels of CEA and CA19-9 in patient with gastric cancer Kodera Y, Yamamura Y, Torii A, et al . Am J Gastroenterol.1996;91:49-53.
- Carbohydrate antigen 19‐9 is a useful prognostic marker in esophagogastric junction adenocarcinoma Tokunaga Ryuma, Imamura Yu, Nakamura Kenichi, Uchihara Tomoyuki, Ishimoto Takatsugu, Nakagawa Shigeki, Iwatsuki Masaaki, Baba Yoshifumi, Sakamoto Yasuo, Miyamoto Yuji, Yoshida Naoya, Oyama Shinichiro, Shono Takashi, Naoe Hideaki, Saeki Hiroshi, Oki Eiji, Watanabe Masayuki, Sasaki Yutaka, Maehara Yoshihiko, Baba Hideo. Cancer Medicine.2015;4(11). CrossRef
- Carbohydrate antigen 19-9 (CA 19-9) (serum, plasma) The Association for Clinical Biochemistry 2012. Available online ..
- Carcinoembryonic antigen-Wikipedia, the free encyclopedia. Cancer Diagnosis-Information about Cancer-Stanford Cancer Center Retrieved 2008-10-15. Available online: http:/ en.wikipedia.org/wiki/Carcinoembryonic_antigen..
- Comparison of serum cytokeratin-18, CEA and CA 19-9 levels in esophageal and gastric cancers Tuncer I, Dülger A, Uygan I, Öztürk M, Kotan C, Şekeroğlu R. Eastern Journal of Medicine.2004;9:72-78.
- Tumor Markers in Serum of Patients with Primary Squamous Cell Carcinoma of the Esophagus Gion Massimo, Tremolada Carlo, Mione Riccardo, della Palma Paolo, Dittadi Ruggero, Zari Claudio, Nosadini Attilio, Castoro Carlo, Ruol Alberto, Peracchia Alberto, Bruscagnin Giuliano. Tumori Journal.1989;75(5). CrossRef
- Diagnostic value of CA 19-9 and CEA in gastrointestinal pathology Patai A, Heber S, Dobronte Z, Kovacs LG. Orv Hetil.1992;133:1301-1307.
- Simultaneus and plasma immunoreactive CEA in 108 patients undergoing gastroscopy Bunn PA, Cohen ML, Widerlite L, Nugent JL, Matthews MJ, Minna JD. Gastroenterology.1979;76:734-741.
- The clinical information value of an immunoenzyme study of the tumor markers CA- 19-9, CEA and AFP in cancer of the stomach,pancreas colon and rectum Ial’chenko NA, Lagutin VD, Lavik NN, Musin II. Vopr Onkol .1991;:37.
- Carcinoembryonic antigen, CA 19-9 and CA 50 in monitoring human squamous cell carcinoma of the esophagus Munck-Wikland E, Kuylenstierna R, Lindholm J, Wahren B. Anticancer Res.1990;10:703-708.
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2020
Author Details