Tissue Zinc Concentration in Prostate Cancer: Relationship with Prostate Specific Antigen and Gleason Score in a Cohort of Nigerian Men
Download
Abstract
Introduction: Prostate cancer (PCa) is the commonest maliganacy among men of African descent. The possible role of trace elements like Zinc in its aetiogenesis and disease severity remains controversial. This paper aims to identify the relationship between tissue zinc concentration and the occurrence of prostate cancer. And to identify the relationship between the tissue zinc concentration and the disease severity (using the prostate specific antigen (PSA) and Gleason scores (GS)).
Methodology: A cross-sectional study of 41 consecutive consenting men with histologically confirmed PCa and 41 age-matched controls. Toe-nail clippings were obtained from both groups and assayed for zinc using the Particle induced X-ray emission (PIXE) technique. Zinc concentrations were compared between cases and controls. A correlation analysis was performed to determine relationship between Zinc concentration and disease severity (PSA and GS).
Results: Forty one men each were recruited in the Prostate cancer and control groups for this study. PCa group had a mean age of 72.83 ± 7.06 years while the control group was 70.73 ±6.40 years. There was statistically significant higher toe-nail Zn concentration among Prostate cancer patients than controls (P= 0.028, t=2.28, df=40). The mean PSA values among the Prostate cancer group was 46.10 ± 40.62ng/ml and mode GS was 8.There was neither a correlation between toe-nail Zn concentration and serum PSA levels (p=0.386) nor with the GS.
Conclusion: This study found a statistically significant higher toe-nail Zn concentration among men with PCa compared with age-matched controls. There was however no correlation between toe-nail Zn concentration and the serum prostate specific antigen or Gleason score.
Introduction
Prostate Cancer (PCa) is the commonest urologic malignancy [1-2]. The last few decades have witnessed an increase in the burden of PCa globally, an increase that is predominant among men of African decent [1, 3-5]. In Nigeria, PCa is the leading cause of cancer related deaths in men accounting for 11% of all male cancers in Nigerian men [1, 6]. This high mortality is attributed to the fact that greater than 80% of PCa cases in our environment present with advanced or metastatic disease hence most only benefit from palliative androgen ablation therapy [7-8].
Despite intense research on this subject, the aetiology of prostate cancer is still very poorly understood. Postulated aetio-pathogenic factors for PCa include advanced age, genetic factors, chronic prostatitis, exposure to androgens, estrogens, leptin, vitamin D, sexually transmitted infections, dietary fats, obesity, alcohol and deficiency of trace elements like zinc and selenium [9]. The role of trace elements in the occurrence of PCa has been the focus of much research efforts over the last two decades [10-13].
One of such trace elements is Zinc (Zn) which is an essential trace element that is ubiquitous in the human body. It is an endogenous inhibitor of the enzymatic activity of prostate-specific antigen (PSA) which is a key factor responsible for the disturbance of the normal homeostasis of insulin-like growth factor 1 (IGF-1) and known to have a role in aetio-pathogenesis of prostate cancer [14-15]. Zinc is predominantly obtained from diet and it is concentrated in blood, nails, hair and other living tissues including the prostate gland. The prostate gland is known to concentrate Zn up to 10 times more than in other tissues. Studies evaluating Zn levels in patients with PCa showed significantly lower (approx. 2–3-fold) zinc concentrations in the prostatic tissue that are affected by the neoplastic process compared to healthy prostatic tissue in the same subject [15-16]. Hence there is an uneven distribution of Zn in the prostate gland of patients with PCa [15]. Cohort studies have demonstrated that dietary Zn intake or plasma Zn concentrations are inversely associated with cancers and mortality risk [17-18].
Kristal et al found that the intake of Zn supplements was associated with a reduced Pca [19] risk while the Vitamin and Lifestyle (VITAL) cohort study revealed that supplemental Zn intake did not influence Pca risk but reduced risk of advanced Pca [20]. In North Carolina, Wagner et al deduced that areas with lower soil zinc content had higher prevalence of PCa [21]. In contrast however, Zhang et al showed that long-term use of zinc supplements increased the risk of PCa [22].
Studies have also attempted further to demonstrate the effect of the tissue Zn concentration on the severity of PCa by comparing with serum PSA values and Gleason score. Vartsky et al showed an inverse relationship between tissue Zn concentration and Prostate Specific Antigen (PSA) with elevated PSA and reduced prostatic Zn increasing the chances of PCa [23]. Cortesi et al found that the lower the local prostatic Zn concentration, the higher the chances of PCa and the worse the Gleason score [24].
Most of the earlier studies in this dormain made use of prostatic tissue to assess tissue Zn concentration and found there is uneven distribution of Zn in malignant prostatic tissue. This study therefore aims to assess toe-nail clippings for tissue Zn concentration in men with PCa to ensure a reliable representation of tissue Zn concentration. The relative ease of sample collection, the fact that it provides a more stable measure of Zn status than serum and prostatic tissue, coupled with a good correlation of Zn levels assayed years apart, aids its preference [12, 25].
The increasing burden of PCa as well as the more aggressive variants of the disease in men of African descent have necessitated this study on the role of Zn in the aetiopathogenesis of PCa and its correlation with PSA and gleason’s score. This study was aimed to determine the correlation between toe-nail Zn concentration, Serum PSA values and Gleason score in patients with Prostate cancer.
Materials and Methods
Patients
This was a prospective hospital-based comparative study conducted in the urology unit of the Obafemi Awolowo University Teaching Hospitals Complex which is a Federal tertiary institution in Ile-Ife, Osun state, South-western Nigeria over a 1-year (September 2017- August 2018). Consecutive patients with histopathologically confirmed prostate cancer following prostate biopsy were recruited. Also age-matched controls without prostate cancer were recruited for comparison. The histological appraisal included the gleason score. A single pathologist was responsible for appraisal of all prostatic biopsy specimens during the study period in order to avoid inter-observer variations.
Exclusion criteria included:
1. Patients who were found to be on Zinc supplements.
2. Patients who had small bowel resection or any bowel
pathology which may affect zinc absorption.
3. Patients who were indulging in application of toe-nail polish or treatment which alter toe-nail zinc concentration.
4. Patients with obvious nail diseases which could alter nail zinc concentration.
5. Patients already on anti-androgens or any form of hormonal ablation
Sample Size Determination
The Leslie-Fischer’s formula for minimum sample size estimation was used to determine the sample size for this study [26-27]:
n= z2 pq/d2
Where:
n = Minimum sample size
z = This is 1.96, when the confidence interval is 95%
p = Estimated prevalence of prostate cancer in our community.
A 5-year review of the hospital day-case surgery records between January 2009 and December 2013, revealed that of a total of 5760 procedures/surgeries, 650 prostate biopsies were done and 144 cases were histologically diagnosed with prostate cancer during this period. Hence a prevalence of 2.5% of the total patients seen during this period was recorded.
Thus, p = 0.025
q = 1.0 – p
= 1.0 –0.025 = 0.975
d = Desired maximum allowable margin of error.
A maximum of
5% margin of error will be allowed in this study Therefore,
n = (1.96)2 × 0. 025 × 0.975/ (0.05)2=37
Considering an attrition rate of 10%, the total sample size became:
N = 100 × 37/ 90 = 41 patients
Thus, a sample size of 41patients was used for cases and 41for controls.
Methodology
Patients presenting to the out-patient clinic with signs and symptoms suggestive of prostate cancer had thorough history taken, general physical examination (including DRE) and investigations (including PSA) done. Patients suspected to have PCa were subjected to extended sextant trans-rectal digitally guided prostate biopsy under caudal anaesthesia (85% of prostate biopsies during the study period were done by the researcher). Prostatic biopsy specimens were transported to the histopathology laboratory in 10% buffered formalin solution. A designated Senior registrar under the supervision of a consultant was responsible for evaluating ALL prostatic specimens to confirm PCa and assign Gleason scores. Individuals with histological confirmation of prostate cancer were recruited for this study. The subject information sheet (Appendix I) was administered to the subject and informed consent was obtained before recruitment for this study. Patients’ demographic characteristics, symptoms and investigation results were recorded in a pre-designed proforma (including the Gleason score) (Appendix II).
Consenting subjects had approximately 0.5cm long toe nail excised from the big toe and placed in a labelled plain bottle before storage in the refrigerator at 0-4oC.
Consecutive age-matched males presenting to the urological or general surgery outpatient clinics with a normal DRE finding and normal PSA were also recruited as controls. The controls had their details entered into the same proforma (Appendix II) and subsequently had toe nail excised for Zn assay.
The respective specimen containers were labelled alphabetically in order to ensure confidentiality to the patient/control and blinding to the Zinc analyst.
Flow chart to guide the research activities was followed.
Case files of both cases and controls were labelled to avoid double count.
Laboratory and clinical analysis
The labelled toe-nails were transported to the Centre for Energy Research and Development (CERD), ObafemiAwolowo University, Ile-Ife, where zinc concentrations were determined using Particle-induced X-ray emission (PIXE) technique. The PIXE experiment was performed using a 2.5 MeV proton beam obtained from CERD ion beam analysis facility, a 1.7 MV Pelletron accelerator, model NEC 5SDH.
Washing of nail specimen
The nail specimen were washed to eliminate contaminants which may contain various minute concentrations of trace elements and hence give a false result. Washing was done with room temperature distilled water and dried at room air.
Calibration of PIXE System
The PIXE set-up was calibrated using some pure element standards and NIST geological standard, NBS 278. The International Atomic Energy Agency (IAEA) Animal Bone standard (IAEA-H-5) was used for the determination of the H-value which was subsequently used for the quantification of the nail samples results and also to assure the accuracy of the experimental procedure.
Zinc concentration in Nails
To obtain the Zn concentration in the various samples, the nails were mounted in the sample holder in the scattering chamber and irradiated for 10-20 minutes with the proton beam. The beam spot was 4 mm in diameter and of low current (3-6 nA). A CamberraSi (Li) X-ray detector with associated pulse processing electronics interfaced to a PC were used for the X-ray data acquisition.
The computer code GUPIXWIN was used for the analysis of the PIXE data. The Zn concentrations in the samples were obtained, tabulated and analysed.
Data Analysis
The data was entered and analysed using SPSS statistical software version 20. Univariate analysis was utilized to determine the sociodemographic data of the subjects, using percentages and means (with standard deviation).
The independent T-test was employed to determine if there was any significant difference in the toe-nail zinc concentration between the study group and control using 0.05 level of significance (P < 0.05). The 95% confidence interval was also put in place.
Pearson correlation was used to determine the relationship between tissue Zn concentration and the PSA as well as GS respectively.
Institutional Ethical Committee Approval
This research was performed in accordance with the ethical standards laid down in the 1964 declaration of Helsinki. Approval was obtained from the ethical committee of the Obafemi Awolowo University Teaching Hospitals Complex (Appendix I and Appendix II). Informed consent was also obtained from all suitable patients before being included in the study. The study was conducted at no additional cost to the patient. The cost of tissue Zn assessment was undertaken partly by the investigator with co-sponsorship through the Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife research grant for residents.
Results
Eighty five men underwent Prostate biopsy during the study period out of which forty-one (41; 48.25%) had histologically confirmed prostate cancer and were recruited for this study. Also, forty-one age-matched adult males (controls) were recruited for this study, all of them were suitable for analysis.
Socio-Demographic Characteristics of Study Population
The age distribution of patients in the PCa group showed a range from 56 to 90 years with mean of 72.83± 7.06 years. The peak age incidence in the PCa group was in the 8th decade (71-80 years) (Table 1).
| Variables | Pca Group | Control Group | Statistics |
| Age, years | |||
| Mean ± SD | 72.83± 7.06 | 70.73 ±6.40 | p=0.250 |
| Age distribution (years), n (%) | |||
| 51-60 | 3 (7.3) | 3 (7.3) | |
| 61-70 | 12 (29.3) | 13 (31.7) | |
| 71-80 | 20 (48.8) | 19 (46.4) | t=3.471 |
| 81-90 | 6 (14.6) | 6 (14.6) | p>0.05 |
| Occupation | |||
| Retired | 21 (51.2) | 13 (31.7) | |
| Civil servant | 12 (29.3) | 19 (46.4) | |
| Business man | 6 (14.6) | 9 (21.9) | |
| Clergy | 2 (4.9) | 0 (0) |
Similar to the PCa group, the age range of patients in the control group was between 55 to 88 years with a mean age of 70.73 ±6.40 years. The age distribution of patients in the control group was also statistically similar to those in the study group as depicted in Table 1. There was no statistically significant difference in the ages of the PCa group vs controls (t=3.471, p > 0.05).
Regarding occupational distribution, about half of the patients in the PCa group were retirees while active civil servants, business men and clergy accounted for the rest. All the patients in this study were domicile in the South-western part of Nigeria with 39;95% of the PCa group resident in Ile-Ife and 37:90.2% of the control group resident in Ile-Ife.
Biochemical and Histopathological Characteristics of Study Population
Among the Prostate cancer patients the PSA values ranged from 12.0 to 177 ng/ml with a mean of 46.10 ± 40.62, median 34 ng/ml. As shown in Table 2 about 70% of men with PCa had PSA values greater than 20 ng/ml. On the other hand, the control group had PSA range of 1.8 to 6.5 ng/ml with a mean of 3.88 ± 1.58. There was a statistically significant difference between the mean of the two groups (p=0.001).
| PCa Group | Control Group | |||||
| Minimum | Maximum | Mean | Minimum | Maximum | Mean | |
| PSA (ng/ml) | 12 | 177 | 46.10 ± 40.62 | 1.8 | 6.5 | 3.88 ± 1.58 |
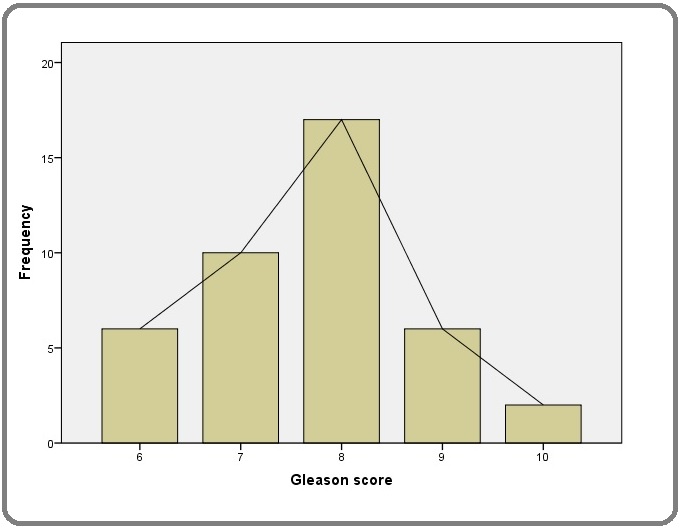
The Gleason score of PCa patients in this study ranged from 6 to 10 with a mode Gleason score of 8. Forty one percent of patients in the study group had a Gleason score of 8 (Figure 1).
Figure 1. Gleason Score Distribution among Men with PCa.
Toe-Nail Zn Concentration among Study Groups
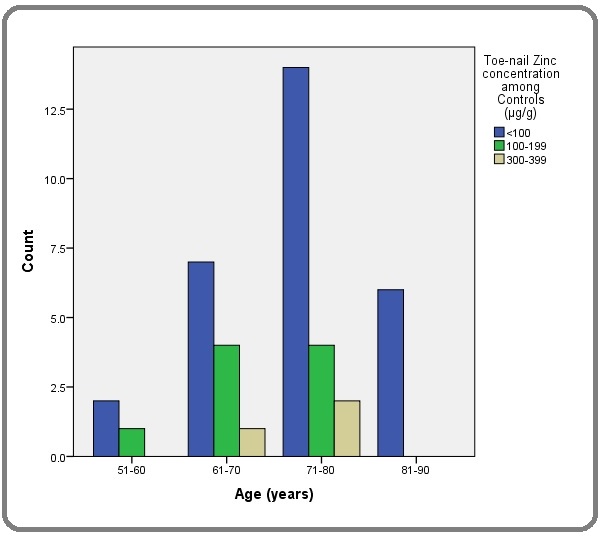
The toe-nail Zn concentration among PCa patients in this study ranged from 38.4 -594.6 μg/g with a median and inter quartile range of 116.5 and 256.47 μg/g respectively while the among the controls the values ranged from 56.7 to 313.8μg/g with a median and inter-quartile range of 96.1 and 29.4μg/g respectively (Figures 2 and 3).
Figure 2. Toe-nail Zinc Concentration Distribution among Men with PCa.
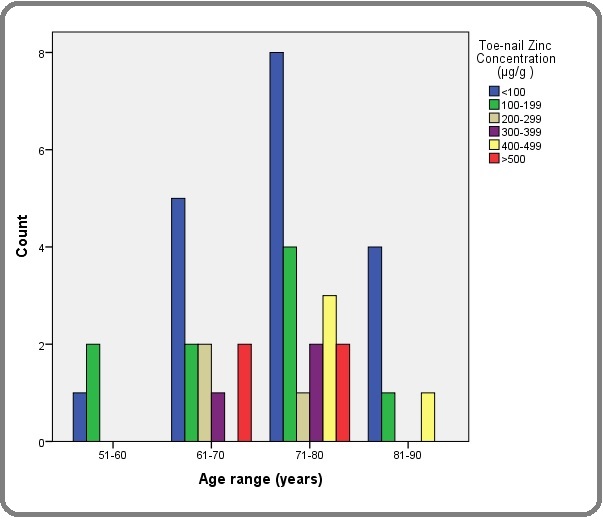
Figure 3. Toe-nail Zinc Concentration Distribution among age-matched Controls.
Eighteen men (44%) with PCa in this study had toe-nail Zn concentration <100 μg/g and the remaining 56% had Zn values of >100 μg/g. Among the controls, 29 men (70.7%) had Zn concentration below 100 μg/g and 12 (29.3%) had Zn concentration >100 μg/g.
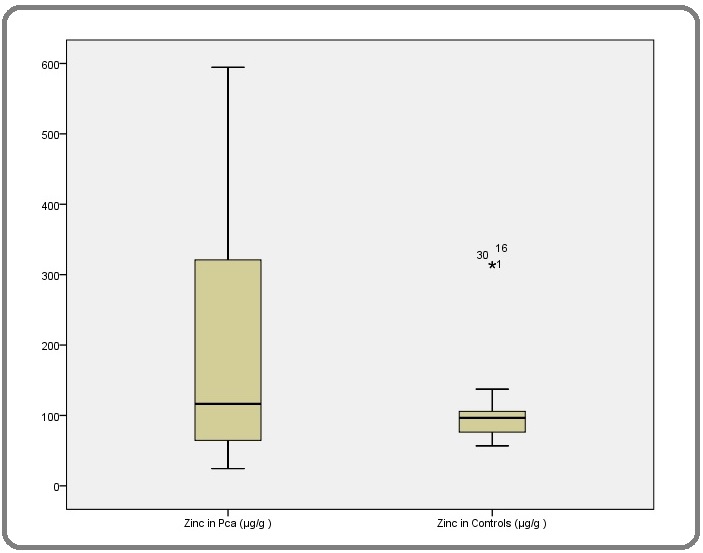
There was statistically significant higher toe-nail Zn concentration among Prostate cancer patients than controls (P= 0.028, t=2.28, df=40) (Figure 4).
Figure 4. Distribution of toe-nail Zinc Concentration among PCa Patients and Controls.
Correlation Of Toe-Nail Zn Concentration With Serum Psa
There was a weak relationship between the toe-nail Zn concentration and the PSA values among men with PCa as depicted in the scatter plot below. Using the Pearson correlation, it was found to be statistically insignificant (p=0.386) (Figure 5).
Figure 5. Relationship of Serum PSA with Toe-nail Zn Concentration in PCa Patients.
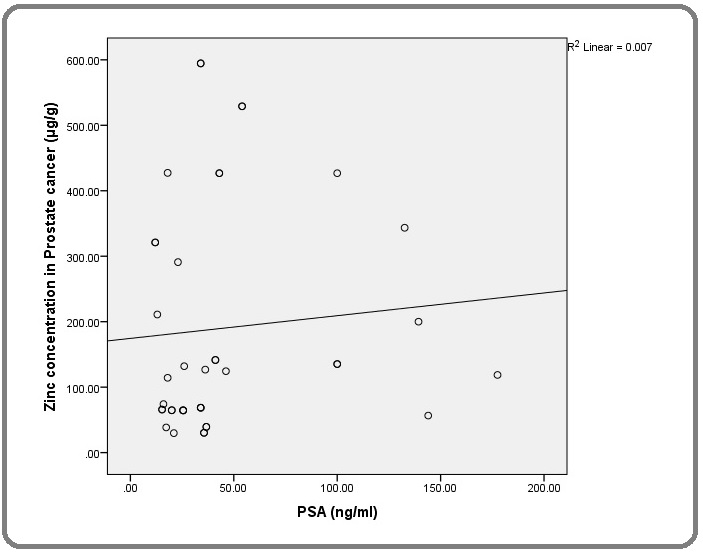
However, among the controls there was a statistically significant correlation between Zn concentration and serum PSA (p=0.04).
Correlation Between Toe-Nail Zn Concentration and Gleason Score
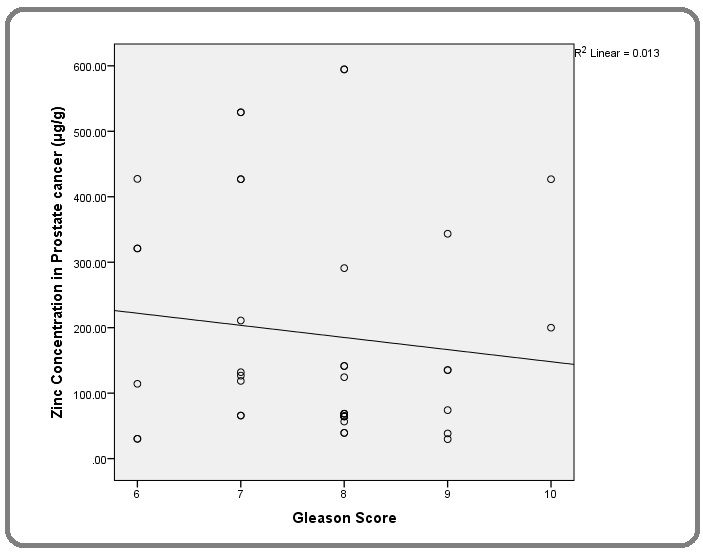
There was an inverse correlation between the toe-nail Zn concentration in PCa patients and their Gleason score in this study. This relationship was however found to be statistically insignificant (p=0.192) (Figure 6).
Figure 6. Relationship of Gleason Score with Toe-nail Zn Concentration in PCa Patients.
Discussion
Prostate cancer is the a leading cause of cancer related mortality in men worldwide. The role of Zn in the aetiogenesis of PCa has been a point of debate for years. This research found that tissue Zinc concentration was significantly higher among men with PCa compared to age-matched controls. There was also no statistically significant correlation between the Zn concentration and the PSA or Gleason score.
The peak age among the study population being between 71 and 80 years validates the fact that Pca is a disease of elderly men as found in other local publications [6, 7, 28]. The succesful age-matching of patients in the two groups as shown by the near equal mean ages reflects the similarity between the groups. It was however noted that PCa could not have been completely excluded among the controls by DRE and PSA only (see limitations). This similarity in age coupled with the fact that men in both groups were domicile in the same part of the country with similar environmental exposure provides the clinical equipoise needed for comparison.
From the findings in this study, there is a comparable toe-nail Zn concentration among the control group and community-based findings by Ayodele et al from Kano in Northern Nigeria [29]. This study further established that the average toe-nail Zn concentration among men with PCa in Ile-Ife was significantly higher than PCa-free age-matched men. This finding may in essence suggest that increased toe-nail and tissue Zn concentration may be a risk factor for PCa. A few international researchers have similarly documenteds increased Zn in diet and tissue as a risk factor for the development of PCa [22, 30-31]. Our findings are further corroborated by researches by Mahmoud et al who found a non-linear increase in risk of prostate cancer with increasing zinc tissue concentration and intake which was not statistically significant among the case-control group. Mahmoud et al in a meta-analysis of 17 studies also showed there was a 24% increase risk of prostate cancer associated with increased zinc intake measured in serum [32] and hair/nail [33].
This study found a marginally positive correllation between tissue Zn concentration and PSA among PCa patients though it was not statistically significant. Considering that there was positive correlation between tissue Zn concentration and PSA values among controls in this study suggests that a longer term follow up of these individuals to identify who develops PCa can be a pointer to Zn either as an aetio-pathogenic agent in PCa or a protective element. Vartsky and colleagues found a positive correlation between tissue Zn concentration and PSa in PCa patients and suggested that combination of these two investigations may increase the specificity of diagnosing PCa without prostate biopsy [23].
Finally, the weak negative correlation between toe- nail Zn concentration and Gleason score may further suggest that among men with PCa, the more poorly differentiated tumours are associated with lower tissue Zn concentration. Zhang et al in their study among a Chinese population found no relationship between tissue Zn concentration and the Gleason score in men with PCa
[22] while Kolonel et al and other researchers have found higher Gleason score among men with high Zn intake and tissue concentration [30-31] although these were carried out among pre-drominantly caucasian populations.
In conclusion, there is a significantly higher toe-nail Zn concentration among men with PCa (median -116.5 μg/g) compared with age-matched controls (96.1 μg/g) in Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife, Osun State (P= 0.028, t=2.28, df=40).
There was a marginal positive correlation between the toe-nail Zn concentration and the PSA values among men with PCa .this was however not statistically insignificant (p=0.386).
Finally, there was a weak inverse correlation between the toe-nail Zn concentration and the Gleason score in men with PCa. This was also statistically insignificant (p=0.192).
Recommendation
1. There is a need for current analysis of the soil and staple food zinc content in Ile-Ife. And also an estimate of zinc consumed in diet among men. This could serve as a background for future recommendations on dietary discretions towards prevention or development of PCa.
2. A longer term follow up of controls with yearly toe-nail Zn assay to identify those that develop Pca in the future may help shed more light on the role of Zn in the aetio-pathogenesis of PCa in a black population.
Acknowledgments
We would like to acknowledge the members of the Urology Unit, Obafemi Awolowo University Teaching Hospitals Complex, Ile-Ife who participated in sample collection and other support during the period of the research. They include Drs Yinka Laoye, Chigozie Onyeze, Rereloluwa Babalola and Ibrahim Akinbola.
References
- Increased Incidence of Prostate Cancer in Nigeria Ogunbiyi JO, Shittu OB. Journal of National Medical Association.1999;91:159-164.
- Histological pattern of prostatic diseases in Nigerians Mohammed AZ, Nwana EJC, Anjorin AS. African Journal of Urology.2005;11(1):33-38.
- Cancer incidence in Nigeria: A report from population-based cancer registries Jedy-Agba Elima, Curado Maria Paula, Ogunbiyi Olufemi, Oga Emmanuel, Fabowale Toyin, Igbinoba Festus, et al . Cancer Epidemiology.2012;36(5). CrossRef
- Burden of prostate cancer in southwestern Nigeria Badmus TA, Adesunkanmi AR, Yusuf BM, Oseni GO, Eziyi AK, Bakare TI, et al . Urology.2010;76:412-416.
- Prostate cancer in Nigerians: facts and nonfacts Osegbe DN. The Journal of urology.1997;157:1340-1343.
- Prevalence and characteristics of prostate cancer among participants of a community-based screening in Nigeria using serum prostate specific antigen and digital rectal examination Ikuerowo Stephen Odunayo, Omisanjo Olufunmilade Akinfolarin, Bioku Muftau Jimoh, Ajala Michael Olawale, Nonyelim Victor Patrick, Esho Julius Olusanmi. Pan African Medical Journal.2013;15. CrossRef
- Emergent trends in the reported incidence of prostate cancer in Nigeria Ifere Godwin, Abebe , Ananaba . Clinical Epidemiology.2012. CrossRef
- Incidental carcinoma of the prostate gland presenting with initial manifestation of disseminated intravascular coagulopathy (dic) in a middle aged man: a case report Salako Ayo, Arowolo Olukayode, Omonisi Emmanuel, Adisa Adewale, Titiloye Nicholas, Adelusola Kayode. Cases Journal.2009;2(1). CrossRef
- Campbell-Walsh Urology 9th Edition Wein AJ, Kavoissi LR, Novick AC, Partin AW, Peters CA. Philadelphia, USA: SAUNDERS ELSEVIER.2007.
- Zinc Intake From Supplements and Diet and Prostate Cancer Gonzalez Alejandro, Peters Ulrike, Lampe Johanna W., White Emily. Nutrition and Cancer.2009;61(2). CrossRef
- Correlation of concentrations of selected trace elements with Gleason grade of prostate tissues Banas A., Kwiatek W. M., Banas K., Gajda M., Pawlicki B., Cichocki T.. JBIC Journal of Biological Inorganic Chemistry.2010;15(7). CrossRef
- Correlates of Toenail Zinc in a Free-Living U.S. Population Gonzalez Alejandro, Peters Ulrike, Lampe Johanna W., Satia Jessie A., White Emily. Annals of Epidemiology.2008;18(1). CrossRef
- Zinc transporters in prostate cancer Franz M.-C., Anderle P., Bürzle M., Suzuki Y., Freeman M.R., Hediger M.A., Kovacs G.. Molecular Aspects of Medicine.2013;34(2-3). CrossRef
- Growth Regulation of Prostatic Stromal Cells by Prostate-Specific Antigen Sutkowski D. M., Goode R. L., Baniel J., Teater C., Cohen P., McNulty A. M., Hsiung H. M., Becker G. W., Neubauer B. L.. JNCI Journal of the National Cancer Institute.1999;91(19). CrossRef
- The correlation between zinc and insulin-like growth factor 1 (IGF-1), its binding protein (IGFBP-3) and prostate-specific antigen (PSA) in prostate cancer Daragó Adam, Sapota Andrzej, Matych Józef, Nasiadek Marzenna, Skrzypińska-Gawrysiak Małgorzata, Kilanowicz Anna. Clinical Chemistry and Laboratory Medicine.2011;49(10). CrossRef
- Disturbed homeostasis of zinc and other essential elements in the prostate gland dependent on the character of pathological lesions Sapota Andrzej, Daragó Adam, Taczalski Jan, Kilanowicz Anna. BioMetals.2009;22(6). CrossRef
- Zinc, Copper, and Magnesium and Risks for All-Cause, Cancer, and Cardiovascular Mortality Leone Nathalie, Courbon Dominique, Ducimetiere Pierre, Zureik Mahmoud. Epidemiology.2006;17(3). CrossRef
- Serum iron, copper and zinc concentrations and risk of cancer mortality in US adults Wu T. Annals of Epidemiology.2004;14(3). CrossRef
- Vitamin and mineral supplement use is associated with reduced risk of prostate cancer Kristal AR, Stanford JL, Cohen JH, Wicklund K, Patterson RE. Cancer Epidemiol Biomarkers Prev.1999;8(10):887-892.
- Zinc Intake From Supplements and Diet and Prostate Cancer Gonzalez Alejandro, Peters Ulrike, Lampe Johanna W., White Emily. Nutrition and Cancer.2009;61(2). CrossRef
- Soil zinc content, groundwater usage, and prostate cancer incidence in South Carolina Wagner Sara E., Burch James B., Hussey Jim, Temples Tom, Bolick-Aldrich Susan, Mosley-Broughton Catishia, Liu Yuan, Hebert James R.. Cancer Causes & Control.2008;20(3). CrossRef
- Vitamin and mineral use and risk of prostate cancer: the case–control surveillance study Zhang Yuqing, Coogan Patricia, Palmer Julie R., Strom Brian L., Rosenberg Lynn. Cancer Causes & Control.2008;20(5). CrossRef
- Prostatic Zinc and Prostate Specific Antigen: An Experimental Evaluation of Their Combined Diagnostic Value VARTSKY D., SHILSTEIN S., BERCOVICH A., HUSZAR M., BRESKIN A., CHECHIK R., KOROTINSKY S., MALNICK S.D., MORIEL E.. Journal of Urology.2003;170(6). CrossRef
- Clinical assessment of the cancer diagnostic value of prostatic zinc: A comprehensive needle-biopsy study Cortesi M., Fridman E., Volkov A., Shilstein S. Sh., Chechik R., Breskin A., et al . The Prostate.2008;68(9). CrossRef
- Toenail trace element levels as biomarkers: Reproducibility over a 6-year period Garland M, Morris JS, Rosner BA, Stampfer MJ, Spate VL, Baskett CJ, et al . Cancer, Epidemiology, Biomarkers & Prevention.1993;2:1-13.
- How to calculate sample size for different study designs in medical research? Charan Jaykaran, Biswas Tamoghna. Indian Journal of Psychological Medicine.2013;35(2). CrossRef
- Relevance of Sample Size Determination in Medical Research Sathian B, Sreedharan J, Baboo SN, Sharan K, Abhilash ES, Rajesh E. Nepal J Epidemiol.2010;1:4-10.
- Cancer of the Prostate: Experience at Nnewi, Southeast, Nigeria Nwofor AME, Oranusi CK. Nigerian journal of clinical practice.2004;7:65-68.
- Cobalt and Zinc in Toenails of Some Kano Inhabitants Ayodele JT, Ajala IC. Nigerian Journal of Basic and Applied Sciences.2011;19(1). CrossRef
- DIET AND PROSTATIC CANCER: A CASE-CONTROL STUDY IN HAWAII1 KOLONEL LAURENCE N., YOSHIZAWA CARL N., HANKIN JEAN H.. American Journal of Epidemiology.1988;127(5). CrossRef
- Zinc Supplement Use and Risk of Prostate Cancer Leitzmann M. F., Stampfer M. J., Wu K., Colditz G. A., Willett W. C., Giovannucci E. L.. JNCI Journal of the National Cancer Institute.2003;95(13). CrossRef
- Antioxidant vitamin and mineral supplementation and prostate cancer prevention in the SU.VI.MAX trial Meyer François, Galan Pilar, Douville Pierre, Bairati Isabelle, Kegle Pastelle, Bertrais Sandrine, Estaquio Carla, Hercberg Serge. International Journal of Cancer.2005;116(2). CrossRef
- Association between Trace Element and Heavy Metal Levels in Hair and Nail with Prostate Cancer Karimi Golgis, Shahar Suzana, Homayouni Nasim, Rajikan Roslee, Bakar Nor Faizah Abu, Othman Mohd Sham. Asian Pacific Journal of Cancer Prevention.2012;13(9). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2021
Author Details