Frequency of Expression of BRAF V600E and Epidermal Growth Factor Receptor (EGFR) in Ameloblastoma
Download
Abstract
Objective: To determine the Immunohistochemical expression of BRAF V600E and Epidermal growth factor receptor (EGFR) in ameloblastoma and correlate the expression with age and gender of patients, and patterns and types of ameloblastoma.
Material & Methods: 39 cases were retrieved with their formalin-fixed, paraffin embedded blocks, trimmed and cut into 5 microns sections. They were mounted on slides after staining with routine hematoxylin and eosin followed by Immunohistochemical staining of BRAF V600E and EGFR. Mean and standard deviation were calculated for quantitative variables. Frequency and percentages were calculated for qualitative variables. Chi-square test was employed to assess the significance of difference. The p-value ≤ 0.05 was considered significant.
Results: There were 19 (41.2%) males and 20 (48.7%) female patients. The mean age of patients at which they presented was 39.97 ± 15.505 (mean ± SD) with an age range between 12 to 65 years. 25 (64.1 %) cases showed positive expression of BRAF V600E and 14 (35.8 %) cases showed negative expression of BRAF V600E. 27 (69.2 %) cases showed positive expression of EGFR whereas 6 (15.3 %) cases showed negative expression of EGFR. The p-value was ≤ 0.05 for expression of BRAF V600E with respect to patterns of ameloblastoma and tumor site and expression of EGFR with respect to sub-types of ameloblastoma.
Conclusion: There is positive expression of BRAF V600E (64.1%) and EGFR (74.4%) in different sub-types and patterns of ameloblastoma. Correct assessment with the help of these markers can lead to early diagnosis and use of adjuvant treatment protocols
Introduction
Odontogenic tumors are the group of heterogeneous tumors among which ameloblastoma is the most common forming 1% of all jaw tumors and cysts [1, 2]. It is reported that they arise from ectomesenchymal, epithelial or mesenchymal cells [3]. Though most common among odontogenic tumors, ameloblastoma is considered as a rare tumor overall [4, 5] with a global incidence of 0.5 cases per million per year [6].
Ameloblastoma grows in a very slow manner having a distinctive infiltrative pattern of growth. It may grow into a malignant tumor in rare cases [7, 8].
Studies have pointed out mutations in genes in MAPK pathway that get affected or dysregulated as the RAF proteins in this pathway may undergo mutations. One of the common mutations in this pathway are of BRAF V600E mutation [9] and changes in epidermal growth factor receptor (EGFR). Recent reports throw light on molecular basis of ameloblastoma similar to BRAF V600E mutation [1,10,11]. Any mutation in EGFR induces cellular processes like migration, proliferation, invasion, angiogenesis as well as apoptosis thus growth of tumor cells [12].
Enough study has not been done to ensure the presence of these mutations in Pakistani population. Therefore, we expect that our study will be useful regarding utilization of immunoexpression of BRAF V600E and Epidermal growth factor receptor (EGFR) in diagnosis of ameloblastoma facilitating and improving post-treatment functional status of patients [13].
WHO has updated the classification of Ameloblastoma in 2017 [14]. In 2017, no type of ameloblastoma was classified as a malignant lesion. According to this classification Ameloblastoma is of four types. Conventional ameloblastoma earlier named as solid or multicystic ameloblastoma [15], unicystic ameloblastomas with one cystic space, metastatic ameloblastoma which is rare but mostly seen in the lung area [7] and peripheral ameloblastoma that develops in gingiva [16]. Almost only two percent of the total ameloblastoma cases and develops in gingiva. In the solid structure two main patterns of tumor are seen i.e. follicular and plexiform. Follicular Pattern is often invasive whereas plexiform pattern is the lesser invasive variant. In the cystic structure there are more cystic spaces seen in both follicular and plexiform pattern. Desmoplastic ameloblastoma is a rare and different variant of ameloblastoma with abundant collagenous/desmoplastic tissue [17]. Acanthomatous ameloblastoma shows squamous metaplasia and stellate reticulum like cells are mostly keratinized. Granular cell ameloblastoma has a granular and eosinophilic cytoplasm and basaloid ameloblastoma shows islands of cells that are basaloid and hyperchromatic without stellate reticulum- like cells [17].
Diagnosis of ameloblastoma is made through radiographs and histology of tumor after the biopsy of tumor is carried out [7, 18].
The most effective treatment for ameloblastoma is resection and enucleation [11]. Often conservative treatments can be opted for removal of ameloblastoma as they can minimize the after effects of surgical resection. Radiation is not successful treatment option for ameloblastoma. Some studies reported that after treating with radiation, a sarcoma was induced [19].
Immunoexpression of BRAF V600E and EGFR in Ameloblastoma
BRAF is a gene which forms protein named as BRAF V600E. This gene plays a role in MAPK pathway which is an important signaling pathway for cell survival and differentiation. BRAF gets mutated in various kinds of tumors, resulting into a change in BRAF V600E protein. Hence, enhancing the spread as well as growth of cancer cells [20]. Many studies have shown presence of BRAF V600E mutation in the ameloblastomas [21-24].
The epidermal growth factor receptor belongs to receptor family of ErbB family. Epidermal growth factor receptor signals essential cell lineage determination and cellular growth, cellular homeostasis, organ morphogenesis, cellular motility and cell survival. When this gene is mutated it results in changes in different cellular processes causing neoplastic changes [1]. Ameloblastoma like many of the head and neck squamous cell carcinomas also show an increased expression of EGFR [2, 25, 26].
Targeted Therapies in treatment of ameloblastoma
As studies show evidence of BRAF V600E and EGFR mutation in variants of ameloblastoma [26-28], the use of targeted therapies was seen as a conservative approach that reduced the need for extensive surgery. These drugs affect different steps along the MAPK pathway performing a selective inhibition of gene and tumor progression. Vemurafenib and debrafenib are two of the most common therapies that prevent the function of the mutated BRAF V600E gene [22, 29]. Tyrosine kinase inhibitors (TKIs) erlotinib, gefitinib, and lapatinib are EGFR inhibitors which function to reduce abnormal growth of tumor [30]. The usage of these conservative therapies for ameloblastoma are still under study as enough data is not yet collected to confirm this approach of treatment [22].
Materials and Methods
Ethical Approval
Ethical approval from the Institutional Review Board (IRB) of AFIP (Armed forces institute of Pathology) was taken to carry out the study. (MP-ORP18-6/READ- IRB/19/646).
Methodology
It was an analytical, cross-sectional study. The study was conducted at the Department of Histopathology, AFIP, and Rawalpindi. Non probability, convenience sampling method was used to collect desired sample size.
Sample Collection
The sample size was 39 cases of ameloblastoma which were retrieved from the records file of Histopathology Department of Armed Forces Institute of Pathology, Rawalpindi, along with their formalin-fixed, paraffin embedded blocks. This study was carried out over a period of one year from June 2019 to June 2020. All fresh cases of ameloblastoma diagnosed on H&E of patients from all ages, groups and both genders were included. All types and patterns of ameloblastomas according to WHO classification 2017 [14] i.e. multicystic, unicystic, peripheral and patterns including plexiform, follicular, acanthomatous, granular and desmoplastic were included in the study. All the specimen with poor fixation, post-chemo radio therapy tissue samples and odontogenic tumors other than ameloblastomas were excluded.
For every case, demographic and clinical details of the patient were recorded in a data collection questionnaire formulated from the histories presented with each case. Consent was taken from all patients in a formulated consent form. Tissue Blocks were trimmed and sliced into small 5 microns sections with the help of a microtome. They were then mounted on slides and stained with routine haematoxylin and eosin followed by immunohistochemical staining.
Immunohistochemical Analysis
Diagnosis was made on H&E slides followed by application of immunomarkers. Indirect method of IHC was used. BRAF V600E Rabbit monoclonal antibody (Clone: RM8 Catalogue no BSB 2829 Isotype: IgG) and EGFR mouse monoclonal antibody (Clone: 31G7, catalogue no: 5473 Isotype: IgG1) were used following their standard protocol of application. Controls were run with each batch for the IHC markers. The microscopic results along with the Immunohistochemical results were conversed and verified by the consultant histopathologist.
Staining evaluation
Staining pattern for BRAF V600E was found in nucleus, cytoplasm and cell membrane whereas for EGFR it was found to be cytoplasmic and membranous. Figure 1 (A & B).
Figure 1. (A & B). Expression of EGFR Showing Membranous Positivity at Different Powers in Plexiform Ameloblastoma. (C & D). Expression of BRAF showing cytoplasmic positivity in cells of follicular ameloblastoma at high power.
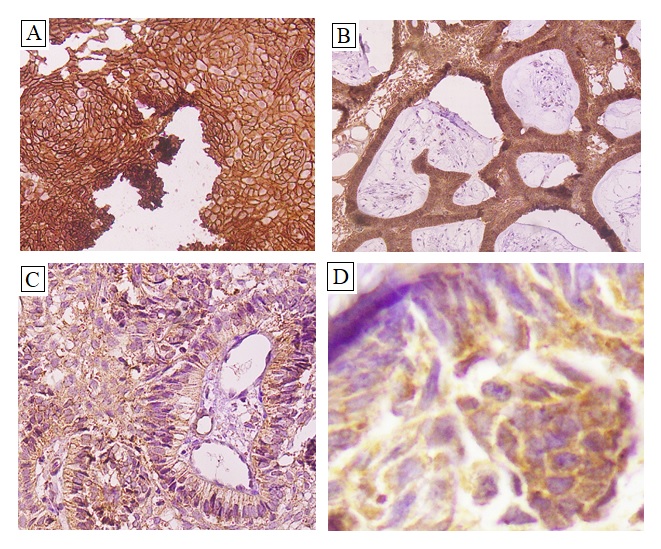
Expression of EGFR showing membranous positivity at different powers in plexiform ameloblastoma. (C & D). Expression of BRAF showing cytoplasmic positivity in cells of follicular ameloblastoma at high power.
Statistical Data Analysis
All the data was collected in the form of qualitative and quantitative variables. The analysis was done in SPSS software version 24.0 with a p-value taken as ≤ 0.05. Quantitative variables like gender, site, subtype, patterns and expression of IHC markers were calculated in frequencies and percentages while mean and standard deviations were taken for qualitative variables such as age. The test of significance (Chi square test) gave the significance of association between variables.
Results
Total number of cases collected were 39. There were 19 (48.7%) male and 20 (51.3%) female patients which corresponded to a male: female ratio of about 1:1.05.
Overall mandible was involved in 32 (82%) of the cases followed by maxilla in 6 (15.4 %) of the cases and 1 case (2.6%) in nose was seen. Mandible remained the affected site in case of all plexiform ameloblastoma (48.7%) followed by acanthomatous ameloblastoma (17.9%).
The overall mean age of the patients at which they presented was 39.97 ± 15.505 (mean ± SD) with the youngest of 12 years and the oldest of 65 years (Table 1).
| Clinicopathological features | N | % |
| Gender | ||
| Male | 19 | 48.7 |
| Female | 20 | 51.3 |
| Age years | ||
| ≤50 | 28 | 72 |
| ≥51 | 11 | 28 |
| Location in jaw | ||
| Mandible | 32 | 82 |
| Maxilla | 6 | 15 |
| Other | 1 | 3 |
| Histologic Variant | ||
| Plexiform | 19 | 48 |
| Acanthomatous | 11 | 27 |
| Follicular | 5 | 13 |
| Granular | 2 | 6 |
| Desmoplastic | 2 | 5 |
| Histologic sub-type | ||
| Multicystic | 30 | 76 |
| Unicystic | 8 | 21 |
| Peripheral | 1 | 3 |
| BRAF V600E IHC | ||
| Positive | 25 | 64 |
| Negative | 14 | 35 |
| EGFR IHC | ||
| Positive | 29 | 74 |
| Negative | 10 | 25 |
19 cases showed plexiform pattern, 11 acanthomatous, 5 follicular, 2 cases were of granular ameloblastoma and 2 cases were of desmoplastic ameloblastoma. Mandible was involved in 32 (82%) of the cases followed by maxilla in 6 (15.4 %) of the cases.
23 (58.9%) cases of solid/multicystic ameloblastoma were found in mandible, 8 (20.5%) unicystic ameloblastoma cases were seen in mandible and only 1 (2.5 %) case of peripheral ameloblastoma was seen in mandible. In maxilla all cases were of multicystic/solid type (15.3 % of total sample size).
Pattern of two immune markers i.e. BRAF V600E and EGFR was then analyzed in these cases (Table 2).
| Histologic patterns of Ameloblastoma (n) | Total | Site of Tumor (n) | |||||||
| Plexiform | Follicular | Acanthomatous | Granular | Desmoplastic | Mandible | Maxilla | |||
| IHC BRAF V600E | Positive | 19 | 3 | 2 | 0 | 1 | 25 | 25 | 0 |
| Negative | 0 | 2 | 9 | 2 | 1 | 14 | 7 | 6 | |
| Total | 19 | 5 | 11 | 2 | 2 | 39 | 32 | 6 | |
| Histologic subtypes of Ameloblastoma | |||||||||
| Conventional/Multicystic | Peripheral | Unicystic | Total | Mandible | Maxilla | ||||
| IHC EGFR Expression | Positive | 27 | 0 | 2 | 29 | 23 | 5 | ||
| Negative | 3 | 1 | 6 | 10 | 9 | 1 | |||
| Total | 30 | 1 | 8 | 39 | 32 | 6 |
64% cases showed positive expression of BRAF V600E. A significant correlation was found between patterns of ameloblastoma and BRAF V600E expression. 17/30 (56.6%) conventional/multicystic solid type showed positive expression of BRAF V600E. 7/8 (87.5%) cases of unicystic ameloblastoma showed positive expression of BRAF V600E. 1/1 (100%) case of peripheral ameloblastoma showed positive expression of BRAF V600E.
All 6 (100%) cases in maxilla showed BRAF V600E negativity. 25 (78.1%) mandibular ameloblastoma showed positive expression of BRAF V600E. A statistical correlation was seen between site of tumor and BRAF V600E expression with a p-value of 0.01.
27 (69.2%) cases showed positive expression of EGFR. Chi square test was applied to compare the expression of EGFR with histological sub-types. P-value of < 0.05 was considered as significant.
Among a total of 19 plexiform ameloblastomas, 11 (57.8%) showed positive expression of EGFR. All 5 (100%) follicular ameloblastoma showed positive expression of EGFR.
The statistical estimates and expression of EGFR with respect to tumor site was not significant.
No correlation was found between the expression of EGFR and BRAF V600E and with gender and age of patient.
Discussion
Ameloblastoma is one of the common tumors among the group of odontogenic tumors. It is generally considered benign in nature. Recent studies have shown that ameloblastoma can often present as a cancerous tumor that has the ability to invade locally [31]. To date, the exact pathogenesis of ameloblastoma is not known. Many studies regard genetics as well as hereditary changes as the cause of tumor development [3, 32].
In this study, the basic clinicopathological features were similar to other such studies. A mean age of 39.97 ± 15.505 was reported as the age of presentation of ameloblastoma which has been confirmed by recent studies where incidence of ameloblastoma was in 3rd - 4th decade [3, 33]. There was no gender predilection [5], [3] (Hendra). The most common pathological pattern of ameloblastoma was plexiform type (48.7%) contrary to other literature work such as done by Gonzalez et al study where follicular type was the predominated type (40%) [22]. This study concluded that most common site for ameloblastoma was mandible (82.1 %) which was relevant to previous studies [3, 4, 34-36]. Other pathological features such as most common sub-type was the conventional multicystic type which was consistent with other studies that showed a prevalence of 56% [33] and 67.7% [35].
It was interesting to note that most of the BRAF V600E and EGFR positive cases were seen in patients less than 50 years of age. Age could be predisposed as a strong, independent and continuous factor in diagnosis of ameloblastoma. This difference could be attributed to genetic, hereditary or ethnic variabilities. Immunoexpression of BRAF V600E was positive in 64% of cases in this study. Previously Oh et al reported a BRAF V600E positivity of 62.4%, [27] while Fregnani et al confirmed a positivity of 46.6%. The increased expression in Pakistani population could be due to racial and genetic factors. This expression was seen in cytoplasm of peripheral palisading cells as well as loosely arranged central cells [24]. This study confirmed BRAF V600E positivity in subtypes such as acanthomatous and desmoplastic type which contradicts the study done by Kelppe et al [37]. Kelppe et al confirmed a negative expression in these two subtypes.
Presence of these two mutations i.e. BRAF V600E and EGFR has often been coincided with each other. [18]. Positive EGFR expression in 74.4 % cases in this study showed the presence of EGFR mutation in MAPK pathway and its critical role in pathogenesis of ameloblastoma. Two recent studies have shown 88% EGFR positivity in cases of ameloblastoma [26, 38] and some studies showed 100% EGFR positivity [8]. An interesting correlation was found between expression of EGFR and types of ameloblastoma with p value of 0.01. No such association were reported in studies done by costa et al and Sanjai et al [26, 38]. Some of the sub-types are therefore predisposed to EGFR mutations.
Positivity of BRAF V600E had a significant correlation with patterns of ameloblastoma consistent with the study done by Shirsat et al that showed a statistically significant association among patterns of ameloblastoma and BRAF V600E immunoexpression [39]. A correlation between site of tumor and BRAF V600E expression depicted positive expression in tumors of mandible. Canto et al reported various significant correlations between different variables and BRAF V600E expression including mandibular location (p =0.0353) and tumor size (P = 0.008) [40]. Similar to our results, Oh et al reported no correlation with variables like age and gender [27]. Positive expression of these markers in various patterns, subtypes and age is useful in further studies regarding diagnosis and pathogenesis of ameloblastoma which is prevalent in south-Asian population. Studies have been done in United states, Europe and Africa that have shown presence of MAPK mutations in ameloblastoma. Lack of data about Pakistani and south Asian population is a limiting factor in the use of inhibitor therapy for MAPK mutations. Morlandt et al demonstrated the use of anti-EGFR therapy for the EGFR positive ameloblastoma. A study done by Kurppa et al. showed significance of EGFR-targeted therapy [13, 41]. The cases of ameloblastoma in which anti EGFR therapy was not effective lead to the discovery of a subset of ameloblastoma which was treated by anti BRAF V600E targeted therapies. Gonzalez et al also reported four case reports with targeted therapy to BRAF V600E [22]. Keeping genetic differences among ethnicities into consideration, use of immune markers as a treatment modality can be studied further to find out the utility of anti BRAF and anti EGFR therapies [20]. However, the small sample size in this study could be a limiting factor in this study and further verifications can be done with a bigger sample size. In conclusion, it was concluded that there is a positive expression of BRAF V600E and EGFR in ameloblastoma occurring in the jaws. A correlation was found between EGFR and histologic sub-types of ameloblastoma. There was no correlation of BRAF V600E and EGFR expression with gender and age of the patient. The positive expression of BRAF V600E and EGFR in ameloblastoma tumor cells may help in treatment protocols and reduce the irreversible post-surgical consequences.
Acknowledgments
I would like to express my profound gratitude to Armed Forces Institute of Pathology (AFIP) for immensely facilitating me during my study period.
Funding Statement
This study was partly funded by National University of Medical Sciences, Rawalpindi and partly by the researcher.
References
- A turning point in therapy for ameloblastomas Abe Masanobu, Zong Liang, Abe Takahiro, Hoshi Kazuto. Oral Oncology.2018;80. CrossRef
- Discovery of novel molecular characteristics and cellular biological properties in ameloblastoma Kondo Sayuri, Ota Akinobu, Ono Takayuki, Karnan Sivasundaram, Wahiduzzaman Md, Hyodo Toshinori, Lutfur Rahman Md, Ito Kunihiro, Furuhashi Akifumi, Hayashi Tomio, Konishi Hiroyuki, Tsuzuki Shinobu, Hosokawa Yoshitaka, Kazaoka Yoshiaki. Cancer Medicine.2020;9(8). CrossRef
- Biological profile of ameloblastoma and its location in the jaw in 1246 Nigerians Agbaje Jimoh Olubanwo, Olumuyiwa Adisa Akinyele, Ivanova Petrova Mariya, Adenike Olusanya Adeola, Osayomi Tolulope, Ajibola Effiom Olajumoke, Oladele Soyele Olujide, Gbenga Omitola Olufemi, Babajide Olawuyi Adetokunbo, Obos Okiti Robinson, Eziafa Saiki Thelma, Fomete Benjamin, Aremu Ibikunle Adebayo, Okwuosa Chuckwubuzor, Abimbola Olajide Mofoluwaso, Mofoluwake Ladeji Adeola, Emmanuel Adebiyi Kehinde, Mobola Emmanuel Mubarak, Sikiru Lawal Hammed, Uwadia Emeke, Oludare Fakuade Babatunde, Mohammed Abdullahi Yusuf, Politis Constantinus. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology.2018;126(5). CrossRef
- Analysis of Prevalence and Clinical Features of Ameloblastoma and its Histopathological Subtypes in Southeast Myanmar and Lower Northern Thailand Populations: A 13-Year Retrospective Study Intapa Chaidan. Journal of Clinical and Diagnostic Research : JCDR.2017;11(1). CrossRef
- Ameloblastoma Masthan K. M. K., Anitha N., Krupaa Jayasri, Manikkam Sudha. Journal of Pharmacy & Bioallied Sciences.2015;7(Suppl 1). CrossRef
- Ameloblastoma: current etiopathological concepts and management Effiom O. A., Ogundana O. M., Akinshipo A. O., Akintoye S. O.. Oral Diseases.2018;24(3). CrossRef
- Cawson's essentials of oral pathology and oral medicine2017 Odell EW, Cawson RA. .
- The Mutational Profile of Unicystic Ameloblastoma Heikinheimo K., Huhtala J.-M., Thiel A., Kurppa K. J., Heikinheimo H., Kovac M., Kragelund C., Warfvinge G., Dawson H., Elenius K., Ristimäki A., Baumhoer D., Morgan P. R.. Journal of Dental Research.2019;98(1). CrossRef
- Immunohistochemistry for Pathologists: Protocols, Pitfalls, and Tips Kim So-Woon, Roh Jin, Park Chan-Sik. Journal of Pathology and Translational Medicine.2016;50(6). CrossRef
- Immunohistochemistry in Investigative and Toxicologic Pathology Janardhan Kyathanahalli S., Jensen Heather, Clayton Natasha P., Herbert Ronald A.. Toxicologic Pathology.2018;46(5). CrossRef
- Recurrent Ameloblastoma: A Surgical Challenge Aramanadka Chithra, Kamath Abhay Taranath, Kudva Adarsh. Case Reports in Dentistry.2018;2018. CrossRef
- A clinicopathologic study of 173 odontogenic tumours in Northern Peninsular Malaysia (2007-2014) Ismail S, Lynn SC. The Malaysian journal of pathology.2018;40(2):129-135.
- High frequency of BRAF V600E mutations in ameloblastoma Kurppa Kari J., Catón Javier, Morgan Peter R., Ristimäki Ari, Ruhin Blandine, Kellokoski Jari, Elenius Klaus, Heikinheimo Kristiina. The Journal of Pathology.2014;232(5). CrossRef
- Update from the 4th Edition of the World Health Organization Classification of Head and Neck Tumours: Odontogenic and Maxillofacial Bone Tumors Wright John M., Vered Marilena. Head and Neck Pathology.2017;11(1). CrossRef
- Radiographic estimation of the growth rate of initially underdiagnosed ameloblastomas Mariz BALA, Andrade BAB, Agostini M, de Almeida OP, Romañach MJ, Jorge J Jr, et al . Oral Medicine and Pathology.2020;24(4):468-472. CrossRef
- A Conservative Approach to a Peripheral Ameloblastoma. Hindawi Publishing Corporatio Borrello R, Bettio E, Bacci C, Valente M, Sivolella S, Mazzoleni S, et al . 2016;5. CrossRef
- Clinicopathological characteristics of desmoplastic ameloblastoma: A systematic review Anand Rahul, Sarode Gargi S., Sarode Sachin C., Reddy Mamatha, Unadkat Hemant V., Mushtaq Shazia, Deshmukh Revati, Choudhary Shakira, Gupta Nitin, Ganjre Anjali P., Patil Shankargouda. Journal of Investigative and Clinical Dentistry.2018;9(1). CrossRef
- Lesions Associated with Impacted Tooth. KDC Baral R, Ojha B, Bajracharya D. 2020;1(1):25-31.
- Malignant Transformation of a Desmoplastic Ameloblastoma to Squamous Cell Carcinoma: A Case Report Rais Rehan, El-Mofty Samir K.. Head and Neck Pathology.2018;13(4). CrossRef
- Persistent response to vemurafenib in metastatic ameloblastoma with BRAF mutation: a case report Broudic-Guibert Morgane, Blay Jean-Yves, Vazquez Léa, Evrard Alexandre, Karanian Marie, Taïeb Sophie, Hoog-Labouret Natalie, Oukhatar Céline Mahier Ait, Boustany-Grenier Rania, Arnaud Antoine. Journal of Medical Case Reports.2019;13(1). CrossRef
- Immunohistochemical analysis of BRAF V600E mutation in ameloblastomas Canto Alan Motta, Silva Marcelino Barbara Michaela Reis, Schussel Juliana Lucena, Wastner Bruna F., Sassi Laurindo Moacir, Corrêa Luciana, Freitas Ronaldo Rodrigues, Hasséus Bengt, Kjeller Göran, Junior Celso Augusto Lemos, Braz-Silva Paulo Henrique. Clinical Oral Investigations.2019;23(2). CrossRef
- Current concepts in ameloblastoma-targeted therapies in B-raf proto-oncogene serine/threonine kinase V600E mutation: Systematic review González-González Rogelio, López-Verdín Sandra, Lavalle-Carrasco Jesús, Molina-Frechero Nelly, Isiordia-Espinoza Mario, Carreón-Burciaga Ramón G., Bologna-Molina Ronell. World Journal of Clinical Oncology.2020;11(1). CrossRef
- Clinical benefit and radiological response with BRAF inhibitor in a patient with recurrent ameloblastoma harboring V600E mutation Fernandes Gustavo S., Girardi Daniel M., Bernardes João Paulo G., Fonseca Felipe P., Fregnani Eduardo R.. BMC cancer.2018;18(1). CrossRef
- BRAF-V600E expression correlates with ameloblastoma aggressiveness Fregnani Eduardo R., Perez Danyel E. da Cruz, Paes de Almeida Oslei, Fonseca Felipe Paiva, Soares Fernando A., Castro-Junior Gilberto, Alves Fábio A.. Histopathology.2017;70(3). CrossRef
- The importance of BRAF-V600E mutation to ameloblastoma metabolism Duarte-Andrade Filipe Fideles, Silva André Myller Barbosa, Vitório Jéssica Gardone, Canuto Gisele André Baptista, Costa Sara Ferreira Santos, Diniz Marina Gonçalves, Fernandes Ana Paula, Toledo Juliano Simões, André Leiliane Coelho, Gomes Carolina Cavaliéri, Gomez Ricardo Santiago, Fonseca Felipe Paiva. Journal of Oral Pathology & Medicine: Official Publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology.2019;48(4). CrossRef
- Expression of SOX2 and EGFR in Ameloblastoma, Odontoameloblastoma and Ameloblastic Carcinoma Sanjai Karpagaselvi, Sangappa Sumana, Shivalingaiah Divya, Kumar Harish, Baker Anjum. Journal of Clinical and Diagnostic Research.2018;12. CrossRef
- High prevalence of BRAF V600E mutations in Korean patients with ameloblastoma: Clinicopathological significance and correlation with epithelial-mesenchymal transition Oh Kyu-Young, Cho Sung-Dae, Yoon Hye-Jung, Lee Jae-Il, Ahn Sun-Ha, Hong Seong-Doo. Journal of Oral Pathology & Medicine: Official Publication of the International Association of Oral Pathologists and the American Academy of Oral Pathology.2019;48(5). CrossRef
- High frequency of BRAF V600E mutation in Iranian population ameloblastomas Derakhshan S., Aminishakib P., Karimi A., Saffar H., Abdollahi A., Mohammadpour H., Kharazi Fard M.-J., Memarha A.. Medicina Oral, Patologia Oral Y Cirugia Bucal.2020;25(4). CrossRef
- Durable treatment of ameloblastoma with single agent BRAFi Re: Clinical and radiographic response with combined BRAF-targeted therapy in stage 4 ameloblastoma Faden Daniel L., Algazi Alain. Journal of the National Cancer Institute.2017;109(1). CrossRef
- Epidermal Growth Factor Receptor Family and its Role in Gastric Cancer Arienti Chiara, Pignatta Sara, Tesei Anna. Frontiers in Oncology.2019;9. CrossRef
- Comparative histological and immunohistochemical study of ameloblastomas and ameloblastic carcinomas Martínez-Martínez Marisol, Mosqueda-Taylor Adalberto, Carlos-Bregni Román, Pires Fabio-Ramoa, Delgado-Azañero Wilson, Neves-Silva Rodrigo, Aldape-Barrios Beatriz, Paes-de Almeida Oslei. Medicina Oral, Patología Oral y Cirugía Bucal.2017;22(3). CrossRef
- Recurrence of Plexiform Ameloblastoma as Acanthomatous Ameloblastoma: A Rare Case Report Bhuyan Sanat Kumar, Bhuyan Ruchi, Sahoo Tapan Kumar, Das Pinali. Contemporary Clinical Dentistry.2019;10(1). CrossRef
- Argument for the conservative management of mandibular ameloblastomas Haq Jahrad, Siddiqui Sarah, McGurk Mark. The British Journal of Oral & Maxillofacial Surgery.2016;54(9). CrossRef
- Acanthomatous ameloblastoma in anterior mandibular region of a young patient: A rare case report Ugrappa Sridevi, Jain Ajay, Fuloria Neeraj Kumar, Fuloria Shivkanya. Annals of African Medicine.2017;16(2). CrossRef
- Global incidence and profile of ameloblastoma: A systematic review and meta-analysis Hendra Faqi Nurdiansyah, Van Cann Ellen M., Helder Marco N., Ruslin Muhammad, Visscher Jan G., Forouzanfar Tymour, Vet Henrica C. W.. Oral Diseases.2020;26(1). CrossRef
- BRAF V600E mutation-specific immunohistochemical analysis in ameloblastomas: a 44-patient cohort study from a single institution Owosho Adepitan A., Ladeji Adeola M., Adebiyi Kehinde E., Olajide Mofoluwaso A., Okoye Ikechukwu S. I., Kehinde Temitope, Nwizu Ngozi N., Summersgill Kurt F.. European archives of oto-rhino-laryngology: official journal of the European Federation of Oto-Rhino-Laryngological Societies (EUFOS): affiliated with the German Society for Oto-Rhino-Laryngology - Head and Neck Surgery.2021;278(8). CrossRef
- BRAF V600E expression in ameloblastomas-A 36-patient cohort from Helsinki University Hospital Kelppe Jetta, Thorén Hanna, Ristimäki Ari, Haglund Caj, Sorsa Timo, Hagström Jaana. Oral Diseases.2019;25(4). CrossRef
- EGFR is not amplified in ameloblastoma Costa V, Fregnani ER, Fonseca FP, Abreu Alves F, Pinto CAL, Kaminagakura E. Oral Surg Oral Med Oral Pathol Oral Radiol.2018;125:454-458. CrossRef
- Low frequency of BRAF V600E immunoexpression in mandibular ameloblastomas: An institutional study Shirsat Pankaj M, Bansal Shivani, Prasad Pooja, Desai Rajiv S. Journal of Oral and Maxillofacial Pathology : JOMFP.2018;22(3). CrossRef
- Recent trends in the treatment of benign odontogenic tumors Covello Paul, Buchbinder Daniel. Current Opinion in Otolaryngology & Head and Neck Surgery.2016;24(4). CrossRef
- Progress towards personalized medicine for ameloblastoma Gomes Carolina C., Diniz Marina G., Gomez Ricardo S.. The Journal of Pathology.2014;232(5). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Biology , 2022
Author Details