Mycosis Fungoides: The Great Masquerader – A Trilogy of Case Reports
Download
Abstract
Mycosis fungoides, also known as granuloma fungoides or Alibert-Bazin syndrome, is the most common form of primary cutaneous T-cell lymphoma. In the early stages of the disease, skin lesions can mimic a variety of other primary cutaneous disorders, including psoriasis, eczema, and tinea infections. Therefore, early suspicion, prompt diagnosis through histopathological and immunohistochemical analysis, and regular follow-up are crucial. This report presented a trilogy of cases demonstrating the varied skin lesions associated with mycosis fungoides.
Introduction
Mycosis fungoides (MF) is a rare entity under Extranodal Non-Hodgkin lymphoma that accounts for 50% of primary cutaneous T-cell lymphoma [1]. First described by French dermatologist Jean-Louis-Marc Alibert, the name arises from the mushroom-shaped fungus shape of the tumour on the skin surface [2]. Mostly seen in the elderly, with a male predominance. Risk factors include smoking, obesity, and certain occupations (petrochemical, textile, and metal industries) [3]. The prevalence of MF is around 5.2 per 100,000 in the western world and is more rare in India [4]. The early course of the disease can manifest as a skin rash, nodules, patches, or plaque-like lesions masquerading as dermatitis, eczema, or psoriasis, thereby leading to a delay in diagnosis, optimal treatment, and the overall life expectancy of the patient [5]. Here we present a trilogy of cases, all with varied skin manifestations, and summarise our approach to diagnosis and treatment.
Case presentations
Case 1
A 52-year-old male presented with progressive hyperpigmented nodules over the face for the last 8 months. The lesions were not associated with any pain, itching, ulcers, sinus, or discharge. There was no lymphadenopathy or hepatosplenomegaly.
Examination showed multiple nodular acneform lesions with diffuse hyperpigmentation all over the face, the largest measuring 1.1cm by 0.75 cm (Figure 1).
Figure 1. A 52-year-old Male with Multiple Nodular Acneform Lesions and Diffuse Hyperpigmentation All Over the Face.

The patient was previously prescribed benzoyl peroxide gel along with clindamycin gel, but without any relief of symptoms. A complete blood count revealed haemoglobin of 12.3 g/dl, a total leukocyte count of 11300/cumm with neutrophil predominance, and a platelet count of 1.2 lakhs/cu mm. Renal and liver function tests were within normal limits. In view of progressive skin lesions without any symptomatic relief with anti-acne medications, a skin biopsy was done, which revealed atypical lymphocytes around the basal layer of epidermis (epidermotropism), which were small to medium type with occasional cells showing cerebriform nuclei. Immunohistochemistry showed cells positive for CD3, CD4, and CD7 and negative for CD2, CD5, CD8, and CD30, with a Mib1 labelling index of 20%. PET CT of the whole body was within normal limits. ISCL and EORTC staging was 1A (T1N0M0B0), and the patient was initiated on psoralen with ultraviolet A (PUVA) therapy of 2.5 J/cm2 twice weekly as an induction with gradual resolution of symptoms.
Case 2
A 45-year-old female presented with multiple erythematous annular skin lesions over the flexor aspect of face, anterior neck, both forearms, anterior abdomen, and both thigh for the last 3 years. The lesions were associated with itching but without any pain, ulcer, sinus, or discharge. Local examination showed multiple itchy, scaly plaque-like lesions on the erythematous base, the largest one on the anterior abdominal wall measuring 12 cm by 8.5 cm. A general examination revealed a BMI of 34 kg/m2 along with bilateral axillary and inguinal lymphadenopathy.
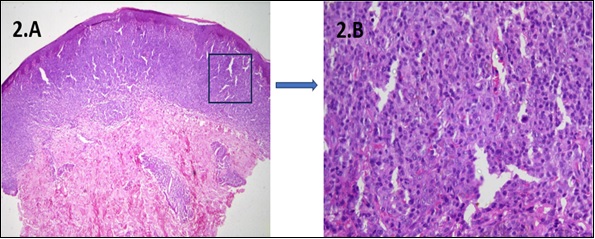
A complete blood count revealed haemoglobin of 11.1 g/dl, a total leukocyte count of 8300/cumm with neutrophil predominance, and a platelet count of 3 lakhs/ cu mm. Renal and liver function tests were within normal limits. Although the lesions resembled psoriasis, in view of concomitant axillary lymphadenopathy, a skin biopsy was done, which revealed the superficial dermis infiltrated by sheets of atypical lymphocytes. The individual cells had moderately pleomorphic convoluted nuclei, fine chromatin, conspicuous nucleoli, and a moderate to scanty amount of cytoplasm. Epidermotropism was present with infiltrate reaching the dermo-epidermal junction, along with pautrier abscesses, which were suggestive of mycosis fungoides (Figure 2). Immunohistochemistry showed cells positive for CD3, CD30, and CD4 and negative for CD2, CD5, and CD7, with a Mib1 labelling index of 40%. PET CT of the whole body revealed metabolically active lymphadenopathy in bilateral axillary (SUV max 4.5) and inguinal regions (SUV max 7). A bone marrow examination revealed normocellular marrow with trilineage hematopoiesis without any infiltration by atypical lymphoid cells. ISCL/EORTC staging [6] was IIB (T3N2M0B0), and the patient was initiated on psoralen with ultraviolet A (PUVA) therapy (4 J/cm2) twice weekly along with topical clobetasol cream and two cycles CVP (cyclophosphamide, vincristine, prednisolone) chemotherapy as induction with gradual resolution of symptoms after 2 cycles.
Case 3
A 58-year-old male presented with a progressive erythematous annular itchy skin lesion over the anterior aspect of the abdomen for the last 2 years. He had been a chronic smoker for the last 15 years, with a history of off-the-counter betamethasone use in view of dermatitis but without any relief. Local examination showed a large erythematous annular plaque measuring 12 cm by 8.5 cm on the anterior abdominal wall. There was no lesion in any other part of the body, lymphadenopathy or organomegaly. A complete blood count revealed haemoglobin of 13.2 g/dl, a total leukocyte count of 5560/cumm with neutrophil predominance, and a platelet count of 2.5 lakhs/cu mm. Renal and liver function tests were within normal limits. A skin biopsy was done in view of the progressive lesion without any relief with anti-eczema therapy. Histopathological examination showed atypical lymphocytes invading the epidermis (epidermotropism), dermis, and cutis without spongiosis. There were occasional cells with cerebriform nuclei along with focal pautrier micro-abscesses suggestive of mycosis fungoides (Figure 2).
Figure 2. A Skin Biopsy of a 45-year-old Female Presented with Multiple Erythematous Annular Skin Lesions Showing 2. A) LOW POWER: Superficial dermis infiltrated by sheets of atypical lymphocytes (epidermotropism) along with pautrier abscesses 2.B) HIGH POWER: The individual cells had moderately pleomorphic convoluted nuclei, fine chromatin, conspicuous nucleoli, and a moderate to scanty amount of cytoplasm.
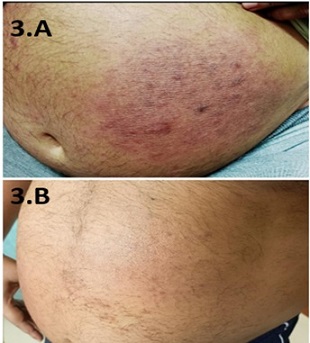
Immunohistochemistry showed cells positive for CD3, CD4, CD5, and CD20 and negative for CD2, CD7, CD5, and CD30 with MIBI 10%. PET CT of the whole body showed no metabolically active disease. ISCL and EORTC staging was 1A (T1N0M0B0), and the patient was initiated on psoralen with ultraviolet A (PUVA) therapy (3 J/cm2) twice weekly as induction, with complete disappearance of the lesion after 12 sessions (Figures 3.A and 3.B).
Figure 3. A 58-year-old Male with a Large Erythematous Annular Plaque on the Anterior Abdominal Wall 3. A) At the presentation 3. B) After 12 sessions of PUVA therapy.
Discussion
Cutaneous T-cell lymphomas are the second most common form of Extranodal Non-Hodgkin lymphoma, of which mycosis fungoides is the most common form. It is a rare form of Non-Hodgkin lymphoma, accounting for less than 1% of cases [7]. It originates from peripheral epidermotropic memory T-cells (CD45RO+) expressing T-cell receptors (TCR) and CD4+.
Incidence increases with age, and the median age lies around 50–60 years with male predominance. Risk factors are poorly described, but common associations include smoking, obesity, and certain occupations (petrochemical, textile, and metal industries). In our trilogy, two of the three were obese and over 50 years of age [8]. Clinically, it manifests in three stages, A) Patch stage: which manifests as erythematous scaly lesions commonly seen in the trunk, gluteal region, and proximal extremities. This is the earliest stage and is commonly misdiagnosed as eczema or psoriasis. B) Plaque stage: in which lesions grow in size and can affect the face and scalp; C) Tumour stage: which manifests as giant nodules that can become ulcerated and necrotic in severe cases. Rare cases may present as hypopigmented, purpuric, vesicular, poikilodermatous, or synringotropic lesions [9]. In the present series, case 1 was in the tumour stage, whereas cases 2 and 3 were in the patch stage. Zarami et al. [10] described two cases of MF, both female, one with a nodular presentation and the other with maculopapular lesions. However, Purnamasidhi et al. [11] described a case report of a 42-year-old male who presented with an ulcerated and necrotic mass in the left gluteal region. Pallazola et al. [12] reported a case of a 58-year-old male with various scattered hyperpigmented patches and plaques, punched-out ulcerations, and subcutaneous nodules in different stages of skin lesions all over the body. A definitive diagnosis is made by skin biopsy. Histopathological examination shows band-like small to medium-sized atypical lymphoid infiltrates in the epidermis (epidermotropism), typically in the basal layer, often as haloed cells. Characteristic cells with cerebriform nuclei and intraepidermal collections (Pautrier’s microabscess) are only seen in a few cases [13]. Immunohistochemistry shows cells positive for CD3, CD4, with a lack of CD2, CD5, and/or CD7 and CD8 expression, which is highly specific for MF and differentiates it from inflammatory disorders such as eczema or psoriasis. TCR beta is positive, whereas gamma is negative, thus differentiating it from gamma delta T-cell neoplasm. The Mib1 labelling index is usually low, and CD30 is negative in earlier stages of disea. WHO clinical variants include folliculotropic type and granulomatous slant skin types, which show CD4 positivity, and pagetoid reticulosis type with CD8 positivity [14]. Staging is done as per ICSL and EORTIC criteria [6].
Treatment of limited-stage disease (stage IIA or below) includes topical therapy (steroids, nitrogen mustards), PUVA therapy, or local radiation. More extensive lesions usually require low-dose chemotherapy (methotrexate, retinoids, interferon, or histone deacetylase inhibitors) in adjunct with total skin electron beam therapy. Advanced-stage disease usually requires a combination of skin-directed therapies, systemic chemotherapy (CHOP, CHOEP, and CVP), and chemoimmunotherapy (alemtuzumab, mogamulizumab, and brentuximab) [15]. Cases 1 and 3 were localised diseases, hence treated with PUVA therapy only, and CVP chemotherapy was added in case 2 in view of extensive skin lesions. All three cases showed excellent responses.
The prognosis of MF patients declines with age, with a 95% 10-year survival rate for early-stage disease and only 3–4 years for advanced disease; hence, early diagnosis is of utmost importance [16].
In conclusion, Mycosis fungoides, a form of cutaneous T-cell lymphoma, is a rare malignant skin condition. Early diagnosis is difficult as early forms have significant overlap with common skin conditions. Hence, regular follow-up along with a skin biopsy is of utmost importance for a timely diagnosis. Immunohistochemical evidence of cells positive for CD3, CD4 with a lack of CD2, CD5, and/or CD7, CD8 expression differentiates MF from inflammatory disorders such as eczema or psoriasis. PUVA remains the mainstay of therapy for localised skin lesions and has an excellent prognosis when initiated early. The addition of low-dose methotrexate improves outcomes with more extensive lesions.
Aknowledgements
I would like to thank my parents Suchitra Roy and Jiban Krishna Roy for their constant support, my professor Dr. Prakas Kumar Mandal for his guidance. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The authors declare no conflict of interest.
References
- Lymphoma classification update: T-cell lymphomas, Hodgkin lymphomas, and histiocytic/dendritic cell neoplasms Jiang M, Bennani NN , Feldman AL . Expert Review of Hematology.2017;10(3). CrossRef
- Mycosis fungoides and the Sézary syndrome: pathology, staging, and treatment Hoppe RT , Wood GS , Abel EA . Current Problems in Cancer.1990;14(6). CrossRef
- Medical history, lifestyle, family history, and occupational risk factors for mycosis fungoides and Sézary syndrome: the InterLymph Non-Hodgkin Lymphoma Subtypes Project Aschebrook-Kilfoy B, Cocco P, La Vecchia C, Chang ET , Vajdic CM , Kadin ME , Spinelli JJ , et al . Journal of the National Cancer Institute. Monographs.2014;2014(48). CrossRef
- Real-Life Barriers to Diagnosis of Early Mycosis Fungoides: An International Expert Panel Discussion Hodak E, Geskin L, Guenova E, Ortiz-Romero PL , Willemze R, Zheng J, Cowan R, et al . American Journal of Clinical Dermatology.2023;24(1). CrossRef
- An illustrated guide to skin lymphomas Cerroni G, Lorenzo N, Kevin L, Gatter F, Helmut K. Jounal of Medicine.2005;12(4):245-263.
- Revisions to the staging and classification of mycosis fungoides and Sezary syndrome: a proposal of the International Society for Cutaneous Lymphomas (ISCL) and the cutaneous lymphoma task force of the European Organization of Research and Treatment of Cancer (EORTC) Olsen E, Vonderheid E, Pimpinelli N, Willemze R, Kim Y, Knobler R, Zackheim H, et al . Blood.2007;110(6). CrossRef
- Skin lymphoma: the illustrated guide Cerroni L. John Wiley & Sons.2020 Jul 14.
- Clinical and epidemiological profile of patients with early stage mycosis fungoides Amorim GM , Niemeyer-Corbellin JPi , Quintella DC , Cuzzi T, Ramos-e-Silva M. Anais Brasileiros de Dermatologia.2018;93(4). CrossRef
- The diagnosis, staging, and treatment options for mycosis fungoides Keehn CA , Belongie IP , Shistik G, Fenske NA , Glass LF . Cancer Control: Journal of the Moffitt Cancer Center.2007;14(2). CrossRef
- Mycosis fungoides: A report of two cases Zarami BA . Int J Case Rep Images.2018;:9:100895Z01BZ2018.
- Mycosis Fungoides: A Case Report Purnamasidhi CAW , Rena NMRA , Suega K. International Journal of Medical Reviews and Case Reports.2019;3(5). CrossRef
- An Elusive Case of Mycosis Fungoides: Case Report and Review of the Literature Pallazola VA , Deib G, Abha S, Geha RM , Kobayashi K. Journal of General Internal Medicine.2019;34(11). CrossRef
- Response to "The WHO classification of haematolymphoid tumours" (Editorial) Swerdlow SH , Campo E, Arber DA , Cazzola M, Cook JR , Döhner H, Dreyling M, et al . Leukemia.2022;36(11). CrossRef
- Mycosis fungoides and Sézary syndrome: 2019 update on diagnosis, risk-stratification, and management Hristov AC , Tejasvi T, Wilcox RA . American Journal of Hematology.2019;94(9). CrossRef
- Management of cutaneous T-cell lymphomas: Established and emergent therapies Wain T, Venning VL , Consuegra G, Fernandez-Peñas P, Wells J. The Australasian Journal of Dermatology.2019;60(3). CrossRef
- Survival, disease progression and prognostic factors in elderly patients with mycosis fungoides and Sézary syndrome: a retrospective analysis of 174 patients Lebowitz E, Geller S, Flores E, Pulitzer M, Horwitz S, Moskowitz A, Kheterpal M, Myskowski PL . Journal of the European Academy of Dermatology and Venereology: JEADV.2019;33(1). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2024
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times