An Audit of Efficacy and Toxicity of Neoadjuvant Paclitaxel and Trastuzumab in HER- 2 Postive Locally Advanced Breast Cancer
Download
Abstract
Background: Her 2 positive Carcinoma breast patients are different subgroup of patients, with poorer disease outcome. Locally advanced Breast cancer (LABC) patients are treated with Neoadjuvant chemotherapy (NACT). Hence with this study we intented to know the efficacy of this Neoadjuvant regimen for Her2 positive LABC.
Materials and Methods: This was a retrospective study including Her 2 positive LABC patients who received NACT with weekly Paclitaxel and Trastuzumab.
Results: Median age of the total 105 patients was 50 years. Except one patient, rest all underwent surgery after NACT. Pathological complete response (p CR) was seen in 40 (38 %) patients. Overall survival at 1 year, 2 years, 3 years and 5 years were 96.2%, 91.3%, 86.6% and 81.4% respectively. In p CR group of patients OS at 1 year and 2 years were 100% and 96.3% respectively , did not change at 3 years and 5 years, whereas in partial response patients OS at 1 year, 2 years, 3 years and 5 years were 93.7%, 85.7%, 77.7% and 70% respectively. Overall DFS at 1 year, 2 years, 3years were 87.4%, 84.3% and 82.1 % respectively.DFS in pCR group at 1 year was 92.3% and it was same at 2years and 3 years, while in p PR group at 1year, 2 years and 3 years were 84.1%, 80.9% and 75.3% respectively.
Conclusion: Neoadjuvant weekly Paclitaxel and trastuzumab combination was well tolerated and resulted in excellent pathological response in Her 2 positive LABC with minimal Grade III/ IV toxicities. Overall DFS and OS was good with significantly better, DFS & OS in p CR group of patients compared to others. Hence NACT with Paclitaxel and Trastuzumab is a well tolerated and effective regimen for Her 2 positive LABC patients. This study shows that p CR translates to better DFS and OS.
Introduction
In India Her 2 over expression is seen in around 16-28% of Carcinoma Breast cases [1]. Her2 positive tumors are associated with poorer survival and clinically aggressive behaviour [2]. Locally advanced breast cancer (LABC) are generally treated with neoadjuvant chemotherapy followed by Surgery and radiotherapy. In Her 2 positive group of patients, Her 2 blockade with targeted therapy have documented improvement in survival. As neoadjuvant treatment, chemotherapy with Her 2 blockade is practised in Her 2 positive breast cancers [3]. With different combination of chemotherapy and trastuzumab as neoadjuvant treatment in Her 2 positive breast cancers, pathological complete response (p CR) rate ranged from 12-45% [4]. Study from Delhi have reported around 36% p CR with Docetaxel, Carboplatin and Trastuzumab in neoadjuvant setting [5].
A Japanese study have showed around 41% p CR with neoadjuvant Paclitaxel and Trastuzumab regimen [6]. To our knowledge there is no published data on the efficacy of neoadjuvant Paclitaxel, Trastuzumab combination from India. Hence with this study we analysed the efficacy in terms of pathological response, survival outcome and toxicity profile of neoadjuvant Paclitaxel, Trastuzumab combination in Her 2 positive Breast Cancers. We also analysed whether pathological response had an impact on survival outcome.
Materials and Methods
Method of Data Collection
Demographic details including age, stage of the disease, side, response to chemotherapy, were collected from case records. Pathological response post neoadjuvant chemotherapy was graded as per CAP (College of American Pathologists) criteria. Grade III/IV toxicity during neoadjuvant chemotherapy were documented. Follow up data of patients were documented from case records.
Our institution NACT protocol for Her2 Neu positive LABC
Inj Paclitaxel 80mg/m2 weekly * 12 cycles + Inj Trastuzumab 4mg/Kg loading dose followed by 2mg/kg weekly (total 9 doses of Trastuzumab).
Operational Definition
Disease free survival (DFS): DFS was calculated from date of surgery to date of disease recurrence.
Overall survival (OS): OS was calculated from date of diagnosis to death due to any cause.
Statistical Analysis
The data was collected and recorded in the designed performa. All analysis was conducted using SPSS version 20.0. The data was expressed as frequencies, percentages, median (IQR). Logistic regression was used to analyse different factors affecting pathological response. Kaplan Meir method was used find the survival data. Log rank was used to compare the survival between p CR and p PR groups.
Results
Total of 105 patient details were analysed. Median age of the patients was 50 years (range 31 to 74 years). Out of the 105 patients, 53 had left sided Ca Breast and 52 had right sided Ca Breast. 36 (34%) patients had some co morbidities. Majority of patients had T3 (50%) as tumor stage and N1 (60%) as nodal stage. Out of 105 patients, 66 (63%) patients had hormone receptor positive disease and 39 (37%) had hormone receptor negative disease. TNM (tumor, node, metastasis) stage wise distribution of patients in table (Table 1).
| T stage | (%) | |
| TX | 1 (1) | |
| T1 | 4 (4) | |
| T2 | 9 (9) | |
| T3 | 53 (50) | |
| T4 | 38 (36) | |
| N stage | ||
| N0 | 11 (11) | |
| N1 | 60 (57) | |
| N2 | 21 (20) | |
| N3 | 13 (12) | |
| M stage | ||
| M0 | 104 (99) | |
| M1 | 1 (1) | |
| Composite stage | ||
| IIA | 1 (1) | |
| IIB | 9 (9) | |
| IIIA | 41 (39) | |
| IIIB | 44 (41) | |
| IIIC | 9 (9) | |
| IV | 1 (1) |
Only 8 patients had Grade III/ IV toxicities. Most common was peripheral neuropathy, in 3 patients. One patient had Grade III neutropenia, one had Grade III diarrhea, one had Grade III hyponatremia and two had cardiac toxicity.
Out of the 105 patients who received, 104 patients had surgery done. 99 patients underwent Modified radical mastectomy, 5 patients underwent Breast conservation surgery and 1 patient did not undergo surgery. Pathological response was documented for 104 patients who underwent surgery. 64 patients had pathological partial response and 40 had complete response. Only 17 patients received adjuvant Trastuzumab. Out of the 17 patients, only 10 patients completed the whole course of 1 year adjuvant Trastuzumab. 95 patients received adjuvant chemotherapy. Pathological complete response in hormone positive group was compared with hormone negative group and was not statistically significant; frequencies are shown in table (Table 2).
| Hormone receptor positive | Hormone receptor negative | P value | |
| Pathological complete response | 35.40% | 42.50% | 0.265 |
Pathological complete response was compared in T stage groups. For comparison purpose T1-T3 cases were grouped as one group and T4 cases were grouped as another group and there was no significant difference, shown in table (Table 3).
| T1-T3 stage | T4 stage | P value | |
| Pathological complete response | 38.80% | 37.80% | 0.547 |
Out of the 105 patients, 89 patients were alive at the time of analysis. Overall survival (OS) at 1 year, 2 years, 3 years and 5 years were 96.2%, 91.3%, 86.6% and 81.4% respectively. Overall survival is shown (Figure 1).
Figure 1. Shows Overall Survival (OS) of Her2 Positive Carcinoma Breast Patients.
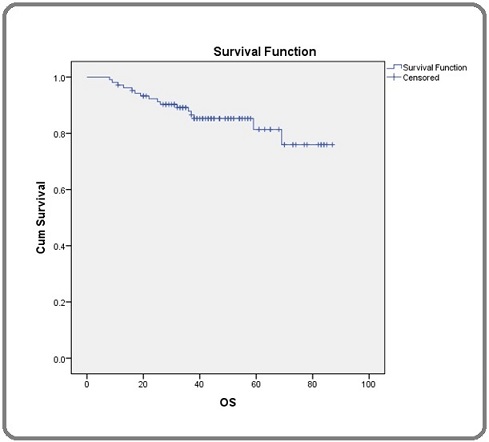
Median follow up time of the patients included in the study was 47 months.
Overall survival of patients with pathological complete response and partial response were compared using log rank test and it was found to be statistically significant with p value of 0.003. Survival curves of complete response and partial response patients are shown separately (Figure 2).
Figure 2. Shows Overall Survival in Patients with Pathological Complete Response (p CR) and Pathological Partial Response (p PR) Separately among Carcinoma Breast Patients.
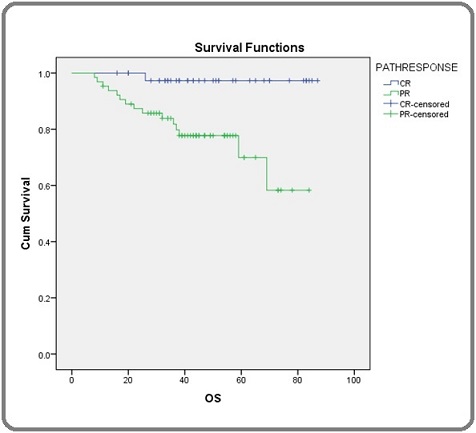
OS for patients who had pathological complete response at 1 year was 100%, at 2 years was 96.3% and thereafter it remained same for 3rd and 4th years. In patients with partial response OS at 1year, 2 years, 3 years and 5 years were 93.7%, 85.7%, 77.7% and 70% respectively. Out of the 104 patients whose disease free survival (DFS) were analyzed, 86 patients were disease free at the time of analysis. DFS is shown (Figure 3).
Figure 3. Shows Disease Free Survival (DFS) in Carcinoma Breast Patients.
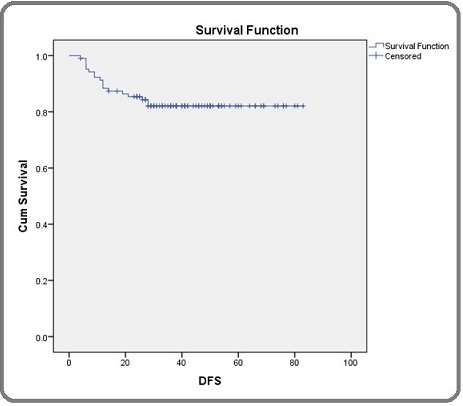
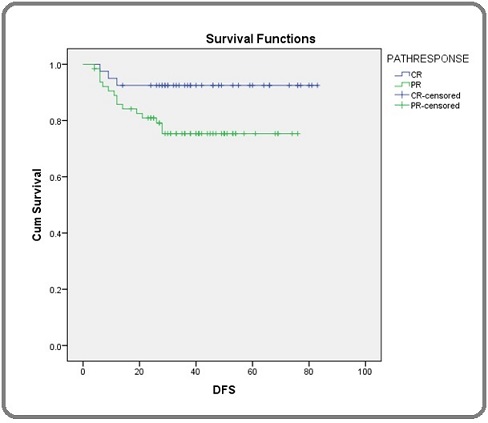
DFS at 1 year, 2 years, 3 years were 87.4%, 84.3% and 82.1 % respectively. DFS of patients who had complete response was 92.5% at 1 year and there was no change at 2 years and 3 years. In partial response patients, DFS at 1 year, 2 years and 3 years were 84.1%, 80.9% and 75.3% respectively. Difference in DFS between the groups was statistically different with a p value of 0.037. DFS of patients with p CR and partial response (p PR) are shown separately (Figure 4).
Figure 4. Shows Disease free Survival (DFS) Patients with Pathological Complete Response (p CR) and Pathological Partial Response (p PR) Separately among Carcinoma Breast Patients.
Discussion
In this study, details of Her2 positive LABC patients who received neoadjuvant chemotherapy (NACT) with Paclitaxel and Trastuzumab were analysed. Total of 105 patient details were analysed. One patient did not undergo surgical intervention, since patient was not willing for surgery. Pathological response was documented for the 104 patients who underwent surgery. Overall the complete pathological response rate was 38.4%. It was similar to the study by Gianni et al [7]. Study by Gao et al also reported similar p CR with Epirubicin, Cyclophosphamide followed by Docetaxel, Trastuzumab neoadjuvant chemotherapy. Same study showed a higher p CR with Docetaxel, Caraboplatin and Trastuzumab combination as NACT [8]. Study by Gianni et al reported a p CR rate of 46% with Docetaxel, Pertuzumab and Trastuzumab combination and p CR rate of 29 % with Docetaxel and Trastuzumab combination [9]. Study by Gupta et al in which weekly Paclitaxel was combined with Trastuzumab and Pertuzumab as neoadjuvant therapy showed p CR rate of 41.6% and was almost similar to our study, which used only Trastuzumab along with weekly Paclitaxel. An Italian study which studied the effectiveness of neoadjuvant Trastuzumab and chemotherapy including different chemotherapy schedule reported p CR rate of around 25 % [10]. Our study had an overall 8% Grade III/IV toxicities, compared to around 18% in study by Gupta et al. Most common toxicity in our study was neuropathy, in 3 patients followed by cardiac toxicity in two patients. In study by Gupta et al most common toxicity was haematological toxicity followed by neuropathy [11]. Out of the 66 hormone positive patients, p CR was 35.4% and in hormone negative group p CR was 45.2% and difference was not statistically significant. But in study by Horiguchi et al hormone negative patients had a better p CR rate, but the number of patients in that study were less compared to our study [6]. On comparison between T1-T3 tumor stage and T4 stage patients, p CR rates were 38.8% and 37.8% respectively. Grade III/IV toxicities to NACT occurred in 8 (8%) of patients. Most common toxicity was Grade III neuropathy in 3 patients. After NACT, 95% of patients completed adjuvant chemotherapy with Adriamycin and cyclophosphamide. Only 17 patients (16%) received adjuvant Trastuzumab and out of the 17 patients only 10 patients completed the whole one year adjuvant Trastuzumab. Most of the patients declined adjuvant Trastuzumab due to financial logistics. Overall DFS at1 year, 2 years and 3 years were 87.4% [7]. There was statistically significant difference in DFS between patients who had p CR to NACT and those who had only p PR. DFS in p CR group was 92.3% at 1year and thereafter there was no change at 2 years, 3 years and 4 years. DFS in patients with partial response were 82.5% and 75.3% at 1 and 2 years respectively and thereafter there was no change at 3 and 4 years. Recurrence free survival (RFS) at 5 years in a similar study was 96% versus 79% in p CR and less than p CR group respectively [12]. OS of the entire patient cohort was 96.2%, 91.3%, 86.6% and 81.4% respectively at 1year, 2 years, 3 years and 5 years respectively. OS at 3 years was similar to patients in NOAH trial who received trastuzumab [7]. Patients with p CR had significant better OS. OS of patients with pCR were 100% and 96.3% at 1 year and 2 years respectively and thereafter there was no change at 3rd and 4th years. OS of partial response patients were 93.7%, 85.7%, 77.7%
and 70% respectively at 1 year, 2 years, 3 years and 5 years respectively. 3 year OS was almost same in p CR group in a similar study [13]. But in the same study 3 year OS was slightly higher compared to our study in less than p CR group. All patients in that study received one year adjuvant Trastuzumab, compared to only 16% in our study and that may be the reason for a slightly reduced OS in less than p CR group. As in other similar studies our study also showed better survival outcome for patients with pathological complete response [12, 13].
In conclusion, in Her 2 positive LABC, addition of trastuzumab to Paclitaxel in neoadjuvant setting resulted in excellent pathological complete response rate with minimal toxicity. p CR was not influenced by the hormone receptor positivity status and T stage of the tumor. Only 16 % of patients received adjuvant trastuzumab due to logistics. DFS and OS were good in the entire patient group and there was significantly better, DFS & OS in p CR group of patients compared to others. Hence NACT with Paclitaxel and Trastuzumab is a well tolerated and effective regimen for Her 2 positive LABC patients. This study shows that p CR translates to better DFS and OS.
Acknowledgements
The authors acknowledge Dr Mohandoss for assisting in manuscript preparation and submission.
Funding and Conflict of Interest
The authors declare no conflict of interest. The study did not receive any funding.
References
- Breast cancer: An overview of published Indian data Rangarajan B, Shet T, Wadasadawala T, Nair NS , Sairam RM , Hingmire SS , Bajpai J. South Asian Journal of Cancer.2016;5(3). CrossRef
- Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer Slamon D. J., Godolphin W., Jones L. A., Holt J. A., Wong S. G., Keith D. E., Levin W. J., et al . Science (New York, N.Y.).1989;244(4905). CrossRef
- Trastuzumab plus adjuvant chemotherapy for human epidermal growth factor receptor 2-positive breast cancer: planned joint analysis of overall survival from NSABP B-31 and NCCTG N9831 Perez EA , Romond EH , Suman VJ , Jeong J, Sledge G, Geyer CE , Martino S, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2014;32(33). CrossRef
- Primary systemic therapy of breast cancer Sachelarie I, Grossbard ML , Chadha M, Feldman S, Ghesani M, Blum RH . The Oncologist.2006;11(6). CrossRef
- Retrospective study of efficacy and safety of neoadjuvant docetaxel, carboplatin, and trastuzumab in HER2-positive locally advanced and oligometastatic breast cancer: An Indian experience Tiwari A., Gogia A., Deo Svs, Shukla N. K., Mathur S., Sharma D. N.. Indian Journal of Cancer.2017;54(1). CrossRef
- Neoadjuvant weekly paclitaxel with and without trastuzumab in locally advanced or metastatic breast cancer Horiguchi J, Oyama T, Koibuchi Y, Yokoe T, Takata D, Ikeda F, Nagaoka H, et al . Anticancer Research.2009;29(2).
- Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort Gianni L, Eiermann W, Semiglazov V, Manikhas A, Lluch A, Tjulandin S, Zambetti M, et al . Lancet (London, England).2010;375(9712). CrossRef
- Neoadjuvant TCH (docetaxel/darboplatin/trastuzumab) versus EC-TH (epirubicin/cyclophosphamide followed by docetaxel/ trastuzumab ) in patients with HER2-positive breast cancer (neoCARH): A randomised, open-label, multicenter, phase II trial. Gao H, Wu Z, Lin Y, Song X, Cao Y, Liu Z, Chen Q, et al . Journal of Clinical Oncology.2020;38(15_suppl). CrossRef
- Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial Gianni L, Pienkowski T, Im Y, Roman L, Tseng L, Liu M, Lluch A, et al . The Lancet. Oncology.2012;13(1). CrossRef
- Neoadjuvant capecitabine and docetaxel (plus trastuzumab): an effective non-anthracycline-based chemotherapy regimen for patients with locally advanced breast cancer Wildiers H., Neven P., Christiaens M. R., Squifflet P., Amant F., Weltens C., Smeets A., et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2011;22(3). CrossRef
- Effect of a combination of pertuzumab, trastuzumab, and weekly paclitaxel on pCR rates and side-effect profile as a neoadjuvant treatment regimen for HER2-positive breast cancer. Gupta NK , Giblin E, Leagre CA , Govert K, Givens S, Chichester T, Locker M, et al . Journal of Clinical Oncology.2018;36(15_suppl). CrossRef
- Pathologic complete response to neoadjuvant chemotherapy with trastuzumab predicts for improved survival in women with HER2-overexpressing breast cancer Kim M. M., Allen P., Gonzalez-Angulo A. M., Woodward W. A., Meric-Bernstam F., Buzdar A. U., Hunt K. K., et al . Annals of Oncology: Official Journal of the European Society for Medical Oncology.2013;24(8). CrossRef
- Pathologic complete response after neoadjuvant chemotherapy plus trastuzumab predicts favorable survival in human epidermal growth factor receptor 2-overexpressing breast cancer: results from the TECHNO trial of the AGO and GBG study groups Untch M, Fasching PA , Konecny GE , Hasmüller S, Lebeau A, Kreienberg R, Camara O, et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2011;29(25). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times