Clinicopathological Spectrum of EBV and MSI in Gastric Adenocarcinomas
Download
Abstract
Introduction: Gastric carcinoma due to Epstein-Barr virus (EBV) and microsatellite instability (MSI) have distinct clinicopathological characteristics that provide specific treatment options.
Material and methods: Patients with gastric adenocarcinomas (GC) diagnosed on treatment-naïve endoscopic biopsy/resection specimens during 19 months were included. The samples were subjected to EBER-ISH and mismatch repair (MMR) protein status by immunohistochemistry and the demographic features, endoscopy/gross features and histologic type were correlated between EBV positive vs negative and MMR-deficient vs proficient groups.
Results: EBV was positive in 3.1% (3/97) of GC. Two of the three patients were males with tumor in the proximal part of the stomach showing poorly cohesive NOS (WHO)/diffuse (Lauren) histology. All the three patients had thickening of the gastric wall and lymph node involvement on CECT and received palliative chemotherapy. MMR-d GC constituted 8% (6/75) of GC. There was female predominance with median age of 53 years. All were in the body of the stomach showing3 each of poorly cohesive (NOS) and tubular adenocarcinoma (WHO)/ intestinal (2), indeterminate (1) and diffuse (3) (Lauren). The loss of MMR expression was seen as heterodimers: MLH1+PMS2 in 04 (66.67%) and MSH2+MSH6 in 01 (16.67%) and isolated loss of PMS2 in 01 (16.67%). Patients received immunotherapy/ palliative chemotherapy. EBV positive and MMR-d GC were mutually exclusive. There was no statistically significant difference in EBV positive vs negative or MMR-d vs proficient groups in clinicopathological features.
Conclusion: Routine testing for EBV by EBER-ISH and MMR by IHC can identify molecularly distinct gastric carcinoma for therapy and prognosis.
Introduction
The incidence of gastric carcinoma varies in different parts of the world and among various ethnic groups. Despite advances in the diagnosis and treatment, the 5-year survival rate of gastric carcinoma is about 20% [1-3]. About 95% of the gastric carcinomas are adenocarcinomas [4]. Gastric adenocarcinoma (GC) is multi-factorial and the classifications based on site and morphology, have limited clinical application for stratification of patients and therapeutic options [5-10].
Gastric carcinogenesis involves multiple genetic and epigenetic alterations of oncogenes, tumor suppressor genes, DNA repair genes, cell cycle regulators and signaling molecules [11]. In a comprehensive molecular evaluation of 295 primary GCs as part of The Cancer Genome Atlas (TCGA) project, a molecular classification was proposed dividing gastric cancer into four sub-types: tumors positive for EBV, microsatellite unstable tumors, genomically stable tumors and tumors with chromosomal instability [12]. Asian Cancer Research Group (ACRG) analyzed the mRNA expression level of 300 tumors and classified them as microsatellite instability-high (MSI-H), microsatellite stable/epithelial-mesenchymal transition (MSS/EMT), microsatellite stable/epithelial/ TP53 intact (MSS/TP53+, p53 active) and microsatellite stable/epithelial/TP53 loss (MSS/TP53-, p53 inactive) [13]. MSS/p53+ molecular subtype was closely linked to EBV infection. Additional subtypes of GC have been described [10, 14-16]. Helicobacter pylori (H. pylori) and Epstein-Barr virus (EBV) are the most well-known pathogens in gastric carcinogenesis [17]. H. pylori is an important risk factor found in 65–80% of primary GCs, particularly non-cardia cancers, while EBV causes about 10% of the GC, particularly cardia cancers [11, 17-18].
Epstein–Barr virus-associated GC (EBVaGC) has several distinct genomic or epigenomic features and clinicopathological characteristics compared with other molecular subtypes of GC. It usually occurs in younger patients with male predominance, in the proximal part of stomach, with less differentiated morphology and high content of CD8+tumor infiltrating lymphocytes and high expression of programmed death-ligand 1 (PD-L1) and PD-L2 [19]. GC with MSI constitutes about 10-22% of GC with distinct clinicopathologic characteristics. It is associated with older age, female gender, distal stomach location, and lower number of lymph-node metastases and a significantly better overall and tumor-specific survival [20].
Molecular classification was considered to be more specific than pathological classification as it allows better stratification of patients. Both EBV-associated and MSI-H-GC are considered to have better prognosis and respond to immunotherapy and hence have the possibility to be useful as biomarkers for patient stratification [20-23]. The gold standard technologies include in- situ- hybridization for the detection of EBV in tissue and polymerase chain reaction (PCR) for MSI status respectively. Immunohistochemistry (IHC) for mismatch repair (MMR) protein has excellent concordance with PCR for MSI status [20].
The present study aims to study the EBV status by in situ hybridization and MSI status by IHC for MMR proteins, in treatment-naïve GC and characterize the clinicopathological subtypes.
Materials and Methods
Patients diagnosed as GC on histology of endoscopic biopsy/resected specimens, prior to chemotherapy/ radiotherapy during the period January 2019 and July 2020 were included in the study. Patients who had tumors other than adenocarcinoma, who received prior treatment, and where blocks were unavailable or had inadequate tissue for further studies were excluded. It was a prospective and retrospective observational study. The study was approved by the institutional ethics committee.
The demographic features, site of the tumor, endoscopic features and/or macroscopic features (on resected specimens) were noted. Features on contrast-enhanced computed tomogram (CECT) were collected wherever available. The hematoxylin & eosin (H&E) stained slides were reviewed and the tumor was classified according to the World Health organization (WHO) (5th) edition criteria as well as Lauren classification [8, 9]. Tumor infiltrating lymphocytes, Crohn-like granuloma or desmoplasia in the stroma were noted and graded as mild, moderate or severe. The adjacent mucosa was examined for chronic gastritis, atrophy, intestinal metaplasia (IM) (on alcian-periodic acid Schiff [APAS] stain) and H pylori status (on Giemsa stain). In situ hybridization (ISH) for EBER ISH for EBV was done using the INFORM EBER (Epstein-Barr Virus Early RNA) Probe implementation kit on VENTANA. Briefly, formalin fixed paraffin embedded (FFPE) sections of 4 μm were deparaffinized followed by 10 min incubation in 3% H2O2. Following rinsing in deionized water (DW), target retrieval was achieved using pepsin digestion in humidity chamber for 15 min. Cell conditioning using standard CC1 (P/N 950-110) at 950C for 44 minutes at pH 8.5 was done. One drop of ISH-PROTEASE 2 was added, coverslip applied and incubated for 8 min. Slides were washed in DW and drained off; 100 μl of INFORM EBER Probe on BenchMark XT probe were poured over each slide, and covered with a cover slip using glue for 1 hour. Red counterstain II, ISH iVIEW Blue Plus Detection Kit, probes, and required accessory reagents onto the reagent tray were loaded. The slides were loaded onto the automated slide stainer and at the completion of the run, slides were dehydrated in graded alcohols, air-dried and mounted with DPX. The intensity of staining (weak, moderate or intense) and the percentage of positive cells were recorded. A section from known EBV positive nasopharyngeal carcinoma was used as control in each batch of the procedure. The results were interpreted as positive staining when there was strong nuclear positivity in the majority of the tumor cells. Nuclear staining in < 5% of tumor cells was considered negative for EBER.
Immunohistochemistry was done using LEICA bio system with monoclonal antibodies: MLH1 (G168-15, prediluted, ready to use), MSH2 (DBM15.82, prediluted, ready to use), MSH6 (44, prediluted, ready to use) and PMS2 (A16-4, prediluted, ready to use). Briefly, FFPE sections of 4µm were deparaffinized, dehydrated and antigen retrieval was done by microwave using citrate buffer/Tris EDTA buffer. After retrieval, slides were cooled to room temperature, washed in distilled water and hydrogen peroxide was added for 10 min. Slides were washed with wash buffer and background snipper was added for 10min. Slides were washed thoroughly thereafter and the sections were treated with primary antibodies against the MMR proteins for 30 min. The reaction product was visualized with diaminobenzidine chromogen (DAB) and counterstaining was done hematoxylin. Only nuclear staining with or without cytoplasmic staining in tumor cells was considered positive (normal expression). Normal gastric mucosa and lymphocytes were used as internal control. Only the complete loss of nuclear staining with positive internal control was considered as loss of MMR protein expression. If any of the MMR protein expression was absent, it was labelled MMR deficient (MMR-d). If all four MMR proteins were expressed, it was labelled as MMR-proficient (MMR-p) [21].
Follow-up details including additional investigations, wherever performed, treatment received and survival were obtained for both EBVaGC and MMR-d GC.
Statistics
The demographic, site and histological parameters were compared between EBV positive and EBV negative GC, and MMR-d and MMR-p GC using Chi square test or Fisher exact test (whichever is appropriate).
Results
A total of 100 patients with tumors diagnosed as GC were included in the study, which comprised 90 endoscopic biopsies and 10 resected specimens. As per the inclusion and exclusion criteria, analysis was performed on 97 patient samples. The resection specimens included 03 total gastrectomies, 02 partial gastrectomies and 05 distal gastrectomies. The demographic features, site, endoscopic features, histologic type of tumor, changes in the stroma and adjacent gastric mucosa were given in Table 1.
| Site | Age (median) years | Gender | Type of Adenocarcinoma (classification) | Tumor stroma | Adjacent gastric mucosa (n=90) | |||||
| M: F | WHO | Lauren | TILs | Desmoplasia | H pylori | IM | Chronic gastritis | Atro gastritis | ||
| Proximal (n =40; 41.24%) | 22-71 [58] | 21:19 | Tubular:13 | Intestinal: 08 | Mild: 18 | Mild: 25 | P: 09 | P:20 | P: 22 | P: 20 |
| Fundus (n=6) | Papillary: 04 | Indeterminate: 09 | Moderate: 11 | Moderate: 09 | N:28 | A:17 | A:13 | A:15 | ||
| Body (n=21) | PC(NOS):14 | Diffuse: 20 | Marked: 11 | Marked: 06 | NA:03 | NA:03 | NA: 05 | NA:05 | ||
| Fundus & body (n=13) | PC(SRC): 06 | Mixed: 03 | ||||||||
| Mixed:03 | ||||||||||
| Distal (n=57; 58.76%) | 28-77 | 38:19:00 | Tubular: 16 | Intestinal: 22 | Mild: 26 | Mild: 32 | P: 14 | P:32 | P: 30 | P:18 |
| Antrum (n=42) | [56] | Papillary:05 | Indeterminate: 04 | Moderate: 14 | Moderate:20 | N:39 | A:21 | A:19 | A:31 | |
| Antrum & body (n=15) | PC(NOS): 12 | Diffuse: 28 | Marked: 17 | Marked:05 | NA:04 | NA:04 | NA:8 | NA:08 | ||
| PC(SRC): 16 | Mixed: 03 | |||||||||
| Mixed: 08 | ||||||||||
| Total (n=97) | 22-77 | 59:38:00 | Tubular:29 | Intestinal: 30 | Mild: 44 | Mild: 57 | P:23 | P:52 | P:52 | P:38 |
| [57] | Papillary: 09 | Indeterminate: 13 | Moderate: 25 | Moderate:29 | N: 67 | N:38 | A:32 | A:46 | ||
| PC (NOS):26 | Diffuse: 48 | Marked: 28 | Marked: 11 | (25.56 %) | NA: 07 | NA: 13 | NA: 13 | |||
| PC (SRC): 22 | Mixed: 06 | NA:07 | ||||||||
| Mixed: 11 |
Abbreviations: WHO, World health organization; PC (SRC), Poorly cohesive signet ring cell; PC(NOS), Poorly cohesive not otherwise specified; TILs, tumor infiltrating lymphocytes; NA, Not assessed; P, present; N, negative; A, absent
Demographic features, site and endoscopic features
There were 59 males and 38 females with age ranging from 22 - 77 (median 57) years. The tumor was located in the proximal part of the stomach in 40 (41.24%; fundus in 06, body in 21 and both fundus and body in 13), distal part in 57 (58.76%; antrum in 42 and antrum and body in 15). The tumor was ulcerated in 46, infiltrative in 6, polypoidal in 4, exophytic in 2, stenotic in 9, gastric wall thickening in 17 and growth (not otherwise specified [NOS]) in 13.
Histologic type of GC
According to the WHO classification, the adenocarcinoma was tubular in 29 (well differentiated in 09, moderately differentiated in 12 and poorly differentiated in 12), papillary in 09, poorly cohesive in 48 (NOS in 26 and signet ring cell [SRC] type in 22) and mixed in 11. Extracellular mucin was present in 09 (9.2%); however, no tumor was categorized as mucinous adenocarcinoma as majority of the samples were endoscopic biopsies and only one resected specimen showed extra cellular mucin but was less than 50% of the tumor. According to the Lauren classification, GC was intestinal in 30, indeterminate in 13, diffuse in 48 and mixed in 6.
Changes in the stroma
Desmoplasia was noted in the stroma which was mild in 57 (58.76%), moderate in 29 (29.89%) and marked in 11 (11.34%). Necrosis was observed in 23 (23.71%). Lymphocytic Infiltration was graded as mild in 44 (45.3%), moderate in 25 (25.77%) and marked in 28 (28.86%). However, Crohn-like reaction was not observed in any tumor.
Changes in the adjacent gastric mucosa
Adjacent gastric mucosa was available in 90 samples. Chronic gastritis was present in 52/84 (61.9%), atrophy in 38/84 (45.2%), IM in 52/90 (53.6%). H.pylori was present in 23/90 (25.56%). Dysplasia was not seen in any sample. Chronic gastritis and atrophy could not be assessed in 6 samples as adjacent mucosa was scant.
EBER-ISH was done in 97 samples and IHC for MMR proteins was done in 75 (as per availability of tissue).
Clinicopathological features of EBV-associated gastric adenocarcinoma (EBVaGC)
It constituted 3.1% (3/97) of GC. The clinicopathological features were given in Table 2.
| EBVaGC | Gender/ Age (y) | Endoscopy/CECT | Histology (classification) | Stroma | Adjacent mucosa | Follow-up | |
| WHO | Lauren | ||||||
| Patient 1 | M/67 | Exophytic, ulcerated bulky growth, 5cm/ circumferential thickening of wall; LN: Paraaortic + | Poorly cohesive NOS | Diffuse | TILs: marked Desmoplasia: mild | H. pylori: N Chronic gastritis: A IM: A Atrophy: A | Received palliative chemotherapy; alive at 2 years follow-up |
| Patient 2 | M/51 | Ulcerated growth along lesser curvature, 1 cm/ asymmetric thickening of wall;LN: Periportal+ | Poorly cohesive NOS | Diffuse | TILs: marked Desmoplasia: mild | H. pylori: N Chronic gastritis: A IM: A Atrophy: A | Received palliative chemotherapy alive at 2 years follow-up |
| Patient 3 | F/77 | Prepyloric encircling growth in antrum, 4x3.5cm/ mild circumferential wall thickening in pylorus; LN: Multiple hepato-gastric, portocaval, peri-pancreatic, retrocaval, aortocaval, pre/para-aortic, mesenteric, bilateral common iliac nodes + | Moderately differentiated tubular adenocarcinoma | Intestinal | TILs: moderate Desmoplasia: mild | H. pylori: P Chr gastritis: P IM: P Atrophy: A | Received palliative chemotherapy; alive at 3 years follow-up |
Abbreviations, EBVaGC, Epstein-Barr virus associated gastric adenocarcinoma; WHO, World health organization; PC(NOS), Poorly cohesive not otherwise specified; TILs, tumor infiltrating lymphocytes; NA, Not assessed; P, present; N, negative; A, absent
Two of the three patients were males with tumor located in the proximal part of the stomach showing poorly cohesive NOS (WHO)/diffuse (Lauren) histology. All the three patients had thickening of the gastric wall and lymph node involvement on CECT. Interestingly, the adjacent mucosa in the moderately differentiated adenocarcinoma (WHO)/intestinal (Lauren) located in the distal part of stomach showed presence of H.pylori, chronic gastritis, IM and atrophy. All the three patients were diagnosed with advanced locoregional disease and received palliative chemotherapy. All patients Please cite figure 1 and 2 in text were alive and at follow-up (2-3 years) and had intact MMR protein expression on IHC.
Figure 1. A, Endoscopic image of EBVaGC showing exophytic growth in fundus and body; Case – 1; B, Photomicrograph H and E 10x gastric adenocarcinoma, diffuse type poorly cohesive NOS; Case – 1; C, Photomicrograph EBER ISH 10x; Case – 1; D, Endoscopic image of EBVaGC showing ulcerated mucosa; Case 2; E, Photomicrograph H and E 10x gastric adenocarcinoma, diffuse type poorly cohesive NOS; Case – 2; F, Photomicrograph EBER ISH 40x; Case – 2; G, Photomicrograph H and E 40x Intestinal type tubular adenocarcinoma; Case – 3; H, Photomicrograph Giemsa 100x H pylori; Case – 3; I, Photomicrograph EBER ISH 40x; Case – 3 .
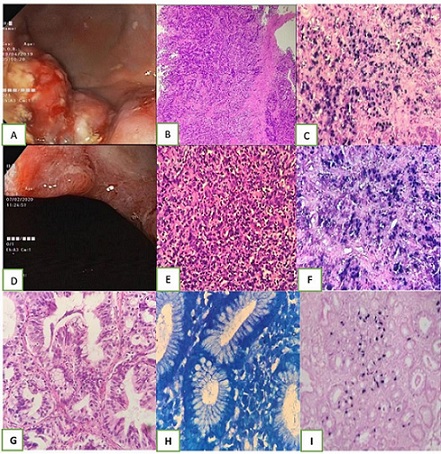
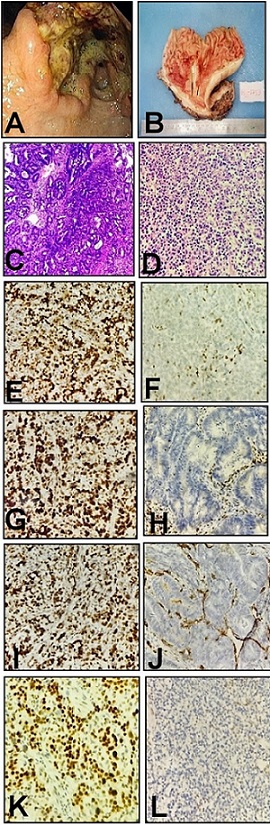
Figure 2. A, Endoscopic image showing ulcero - prolifer- ative growth with everted edges in the antrum of stomach; B, Gross specimen of distal gastrectomy with large nodulo-ulcerative growth in the antrum and extending in to the body of stomach; C and D, Histomorphology in MSI gastric cancers; Tumors demonstrated tubular (C) H and E 40X ,diffuse and solid sheets of tumour cells (D) H and E 10X, with large amounts of tumor-infiltrating or surrounding lymphocytes. E-L) Representative immunohistochemistry shows intact expression of hMLH1 (E), hMSH2 (G), hMSH6 (I) and PMS2 (K), loss of expression of hMLH1 (F), hMSH2 (H), hMSH6 (J) and PMS2 (L). Lymphocytes and stromal cells serve as internal positive control.
Clinicopathological features of MMR deficient-GC
MMR- d GC constituted 8% ( 6/ 75) of GC. The clinicopathological features are given in Table 3.
| MMR-d GC | Gender /Age | Site | Endoscopy/CECT | Histological classification | MMR status | Stroma / Adjacent mucosa | Additional features | Follow-up | |
| WHO | Lauren classification | ||||||||
| Patient 1 | F/26 | Body & fundus | Large cauliflower ulcerated growth/ polypoidal wall thickening; LN: peri-gastric + | Tubular, well differentiated | Intestinal | Loss of MSH2 | TIL: Marked Desmoplasia: Mild H. pylori: N Chronic gastritis: A IM: AAtrophy: A | Significant family history + | Stable |
| Patient 2 | M/57 | Body & fundus | Ulcerated growth along lesser curvature; LN: + | Poorly cohesive NOS | Diffuse | Loss of MLH1, PMS2 | TIL: Marked Desmoplasia: Mild H. pylori: N Chronic gastritis: A IM: AAtrophy: A | NGS: PIK3CA, FGFR2 mt, HER2neu: N | On immunotherapy; stable at 2 years; anastomotic site developed recurrence |
| Patient 3 | F/56 | Body | Ulcerated growth/ wall thickening in body; LN: + | Poorly cohesive NOS | Diffuse | Loss of PMS2 | TIL: Marked Desmoplasia: Moderate H. pylori:N Chronic gastritis: P IM: AAtrophy: P | PDL1 – TPS Score: 30%; HER2neu: N | Palliative chemotherapy; stable at 2 years follow-up |
| Patient 4 | M/59 | Body along greater curvature | Infiltrating growth/PET-CT: irregular heterogeneously enhancing wall thickening; Omental nodule+ | Tubular moderately differentiated | Intestinal | Loss of MLH1, PMS2 | TIL: Moderate Desmoplasia: Mild H. pylori: N Chronic gastritis: A IM: A; Atrophy: A | PDL- TPS: 5% HER 2 neu: N | Palliative chemotherapy, immunotherapy; partial response |
| Patient 5 | F/50 | Body along greater curvature | Ulcerated growth 5 cm/ wall thickening;LN: peri-gastric, hepato-gastric, mesentery of epigastrium and along coeliac and left gastric regions | Tubular, poorly differentiated | Indeterminate | Loss of MLH1, PMS2 | TIL: Marked Desmoplasia: Mild H. pylori: N Chronic gastritis: P IM: A; Atrophy: A | Nil | Radical radiotherapy |
| Patient 6 | F/47 | Body & antrum | Infiltrative growth | Poorly cohesive NOS | Diffuse | Loss of MLH1, PMS2 | TIL: Moderate Desmoplasia: Moderate H. pylori: N Chronic gastritis: P IM: PAtrophy: P | Candida colonization+ | Palliative chemotherapy |
Abbreviations, MMR-d GC, mismatch repair-deficient gastric adenocarcinoma; CECT, contrast-enhanced computed tomogram; WHO, World health organization. PC (NOS), Poorly cohesive not otherwise specified; TILs, tumor infiltrating lymphocytes; NA, Not assessed; P, present; N, negative; A, absent.
There was female predominance with median age of 53 (26-59) years. All the tumors were in the distal part (body) of the stomach. The WHO category was poorly cohesive (NOS) in three, tubular adenocarcinoma (one each of well, moderately and poorly differentiated) in three. According to Lauren classification, the GC was intestinal in 2, indeterminate in one and diffuse in 3. The stroma showed marked lymphocytic infiltration in 4; moderate desmoplasia in 2 and adjacent mucosa showed chronic gastritis in 04, atrophy in 02 and IM in 01. H.pylori was seen in 01 sample.
Loss of MMR expression was observed in 06 (8%) patients. The loss of expression was seen as heterodimers: loss of MLH1+PMS2 in 04 (66.67%) and loss of MSH2+MSH6 in 01 (16.67%). Isolated loss of PMS2 was seen in 01 (16.67%). EBER-ISH was negative in all 6 patients, which were MMR deficient.
Follow-up
All the patients were diagnosed with locally advanced disease with regional lymphadenopathy. One patient had significant family history (mother had endometrial carcinoma). Three patients got investigated further; Her2Neu was negative in all 3; PDLI score was 30% and 5% in two patients tested and one showed partial response; next generation sequencing (NGS) done in one patient showed mutations of PIK3CA, FGFR2. Three patients received palliative chemotherapy, one received radical radiotherapy and one had local recurrence. All patients were alive at 1 year follow-up.
EBVaGC and MMR-d GC
The two molecularly defined GC in the present study, EBVaGC and MMR-d GC were mutually exclusive.
There was no statistically significant difference in the clinicopathological variables between EBVaGC and EBVnGC as well as MMR-d GC and MMR-p GC (Table 4).
| Parameter | EBVaGC (n=03) | EBVnGC | p value |
| (n=94) | |||
| Age (median) years | 67 (51, 67, 77) | 53.4 | |
| Gender (M: F) | 02:01 | 1.54:1 | 0.83 (Chi square test) |
| Site | |||
| Proximal | 2 | 38 | |
| Distal | 1 | 56 | 0.567 (Fisher exact test) |
| Adenocarcinoma histologic type (WHO) | |||
| Tubular | 1 | 28 | 0.564 (Chi square test) |
| Papillary | 0 | 9 | |
| Mixed | 0 | 11 | |
| Poorly cohesive SRC | 0 | 22 | |
| Poorly cohesive NOS | 2 | 26 | |
| Adenocarcinoma histologic type (Lauren) | |||
| Intestinal | 1 | 29 | 0.85 (Chi square test)) |
| Indeterminate | 0 | 13 | |
| Diffuse | 2 | 46 | |
| Mixed | 0 | 6 | |
| Tumor stroma | |||
| TIL | Mild: 0 | Mild: 44 | 0.158 (Fisher exact test) |
| Moderate: 01 | Moderate: 24 | ||
| Marked: 02 | Marked: 26 | ||
| Desmoplasia | Mild: 03 | Mild:54 | |
| Moderate: 0 | Moderate: 29 | 0.686 (Fisher exact test) | |
| Marked: 0 | Marked: 11 | ||
| Adjacent mucosa | |||
| Chronic gastritis | 1/2 | 52/82 | 0.697 (Chi square test) |
| Atrophy | 1/2 | 38/82 | 0.918 (Chi square test) |
| Intestinal metaplasia | 1/3 | 51/87 | 0.38 (Chi square test) |
| H.pylori | 1/3 | 22/87 | 0.75 (Chi square test) |
Abbreviations, MMR-d GC, mismatch repair-deficient gastric adenocarcinoma; MMR-p GC, mismatch repair-proficient gastric adenocarcinoma; CECT, contrast-enhanced computed tomogram; WHO, World health organization; PC(NOS), Poorly cohesive not otherwise specified; TILs, tumor infiltrating lymphocytes; NA, Not assessed; P, present; N, negative; A, absent
Though statistically significant differences were not seen, EBVaGC was more frequently seen in males, in proximal part of stomach with poorly cohesive (NOS) histology and MMR-d GC was more frequently seen in females and distal part of the stomach with equal frequency of poorly cohesive (NOS) and tubular adenocarcinoma. Poorly cohesive (SRC), papillary, mixed and mucinous histology were not seen in both. Both showed moderate to marked tumor infiltrating lymphocytes and minimal to moderate desmoplasia. H.pylori was seen in one sample each with EBVaGC and MMR-d GC. Both were diagnosed with locally advanced disease with regional lymphadenopathy.
Discussion
The present study of GC from a tertiary cancer hospital in south India shows a male predominance with a median age of 57 years and a third (32.9%) of the patients in the age group of 51 to 60 years. The most frequent (58.76%) site of involvement was the distal part of the stomach.
These findings were in agreement with other studies from India and other parts of the world [4, 10, 24-28]. Non-cardia GC was reported to be more commonly associated with H. pylori infection and it is a known causative factor for gastric cancer; H pylori induced chronic inflammation leads to chronic gastritis, followed by atrophic gastritis, IM, dysplasia and invasive tumor (intestinal-type GC according to Lauren classification) [29]. With better prevention of H. pylori infection, the proportion of non-cardia GC are declining and the number of cardia GC is on the rise [4, 27, 30]. H. pylori was seen in 25.56% samples in the present study. Adjacent mucosa showed chronic gastritis in 61.9%, atrophy in 45.2%, IM in 53.6%; however, dysplasia was not identified in any sample. Although infection with H. pylori is very common worldwide, only a small fraction of infected individuals develops GC, implying the role of other factors like smoking, alcohol, environmental and genetic factors [17, 31].
Classification of GC was done according to both Lauren and WHO classifications. The most common histology in the present study was poorly cohesive (WHO)/ diffuse (Lauren) type. Differentiated tubular (WHO)/ intestinal (Lauren)/type was reported to be more common [4]; however, relative proportion depends on the risk factors in a particular geographical region.
Association of EBV with GC: EBV is a ubiquitous herpes virus and infects approximately 90% of the world’s population with no endemic areas. It is well documented to be causally associated with various malignant tumors, including gastric carcinoma. It increases the risk of gastric cancer by 18 times [5, 32, 33]. TCGA proposed a classification based on molecular profiling, which included gastric tumors positive for EBV as a distinct entity [12]. EBVaGC is defined by the presence of EBV in neoplastic cells. The different methods to detect EBV include ISH, different types of PCR assays, and IHC. EBV-encoded small RNAs (EBER1/EBER2) are abundantly produced in the nucleus of latently infected cells (10 6–7 copies per cell) and nearly all cancer cells are positive for EBER-ISH, while adjacent nonneoplastic gastric epithelial cells and infiltrating lymphocytes, other normal stromal cells and foci of intestinal metaplasia are negative [34-38]. Hence, EBER-ISH is considered the gold standard technique for the detection of EBV in tissues with high sensitivity and specificity [34-37]. Tavakoli et al reported that EBV was detected more frequently in biopsy samples than in FFPE specimens from surgically resected GC (2.4-fold, P < 0.0001) due to the low yield of extracted nucleic acids and fragmentation of genomes and transcripts resulting from the processing and hence the authors recommended using biopsy samples to prevent false-negative results.5 In the present study, majority (87/97) were endoscopic biopsies and EBER-ISH was used for the detection of EBV. However, intra-tumoral heterogeneity may hamper detection of EBV status in biopsy samples [39].
The reported frequency of EBER positivity varied from 1.7-10% in various series [5, 10, 13, 20, 40-49]. Tavakoli et al in a meta-analysis, showed that the pooled prevalence of EBV in 20,361 gastric cancer patients was 8.77% (95% CI: 7.73–9.92%; I2 = 83.2%) [5]. Frequency of EBER in our study was 3.09%. The intensity of staining (low to strong) and number of cells stained positive (5 to >80%) were reported to vary [20]. Majority cells with strong intensity of positivity only were considered positive in the present study. However, there is no a clear cut-off for defining positivity in the ISH study for EBER, and most published studies consider the nuclear staining in tumor cells as positive without any reference to the intensity or the percentage of cells [50-52]. The EBV genome in patients with GC is reported to vary in different geographical regions; however, when meta-analysis was restricted to studies that used EBER-ISH for EBV detection, no appreciable difference was seen in geographical prevalence rates and hence it was attributed to method of detection rather than geographical differences [5, 26, 53, 54]. An even higher positivity rate has been reported, when EBV was detected by RNA-Seq instead of traditional EBER1/2 in situ hybridization [55].
EBVaGC is reported to have distinct clinicopathological features; more predominant in men, tends to be located proximally, often a diffuse histologic subtype, and shows a lower frequency of lymph node metastasis than conventional adenocarcinoma [10, 33, 45, 47, 48, 52, 54, 56-58]. Though the number of patients with EBVaGC was small in the present study, 2/3 patients were male and the tumor was in the proximal part of stomach with poorly cohesive NOS/diffuse histology and lymphocytic infiltration in the stroma. Camargo et al. observed that the average age of EBV-positive gastric cancer patients was 58 years old, and 71% of them were men [47]. EBVaGC in its early stage shows a characteristic histology called a ‘lace pattern’ in an intramucosal lesion, which shows irregularly anastomosing tubules and cords associated with moderate to dense lymphocytic infiltration as seen in one of the samples. When this pattern is observed in biopsy specimens, EBER-ISH is recommended for diagnostic purposes [36]. Apart from poorly differentiated/diffuse histology, lymphoepithelioma-like histology and gastric carcinoma with lymphoid stroma were reported to be frequently associated with EBV [57, 59]. The patient who had tumor in distal part of the stomach showed tubular/intestinal histology and was associated with chronic gastritis and H. pylori in the adjacent mucosa. Simultaneous infection with EBV and H pylori can occur and infection with H pylori is related to the occurrence and development of EBV-positive gastric cancer [56]. Similar findings were reported earlier [60, 61]. The distribution of histological subtypes of gastric cancer and the frequencies of H. pylori and EBV associated gastric cancer vary across the globe [62]. EBVaGC were reported to have lymphoid stroma and minimal fibrosis as seen in the present study[63]. Prominent inflammatory infiltrate, particularly CD8-positive or CD4-positive T cells accompanied by CD68-positive histiocytes in the tumor is one of the characteristic features of EBVaGC [36]. This feature reflects the immunogenicity of EBV. EBVaGC was found to express high levels of PD-L1 in cancer and infiltrating immune cells [64, 65]. EBVaGC is reported to have less lymph node involvement and favorable outcome[66]. All three patients in the present study had locally advanced disease with lymph node involvement. It is probably related to presentation at an advanced stage. There was no statistically significant difference between EBVaGC and EBVnGC. Similar observations were made earlier [8, 9, 20, 59].
Tumors positive for EBV, display recurrent PIK3CA mutations, extreme DNA hypermethylation, and amplification of JAK2, CD274 (also known as PD-L1) and PDCD1LG2 (also known as PD-L2) and EBVaGCs seem to be particularly suitable for an anti-PD-L1/PD-1 immune therapy [39, 56]. Thus, detecting EBVaGCs may have clinical implications for considering immune checkpoint therapy [39, 56].
MMR-d GC: Carcinogenesis being a multistep process driven by multiple genetic and epigenetic changes, genetic instability is an important factor. Microsatellites (MS) are short tandem repeats (1–6 nucleotides) scattered through the whole genome, that are prone to a high mutation rate [67]. MSI results from abnormal function of one or more mismatch repair genes (MLH1, PMS2, MSH2, MSH6). Across the gastrointestinal tract, the role of MSI in tumorigenesis has been studied more often in colorectal cancer; however, MSI is one of the pathways implicated in gastric carcinoma. The molecular classification by both TCGA and ACRG identify the MSI subgroup as a specific and well-defined GC entity, associated with favorable outcome [67].
MSI evaluation may be done by IHC, PCR (Fluorescent multiplex PCR and capillary electrophoresis (CE), NGS, single-molecule molecular inversion probes (smMIPs) [68, 69]. PCR testing currently is the most direct, accurate, and cost-effective measurement method. IHC detection of MMR proteins is based on specific antibody recognition of MLH1, MSH2, MSH6, and PMS2 in tumor cell nuclei and is simple, rapid, inexpensive, and requires minimal specialized instrumentation. MSI PCR testing and MMR IHC show an excellent concordance [20]. MMR protein IHC was done in the present study. All samples were treatment-naïve (endoscopy biopsies and resection specimens prior to NACT) as tumors from patients exposed to preoperative chemotherapy or radiation therapy are more difficult to assess using IHC due to artifactual loss of MSH6 protein expression. Disadvantage for IHC is that it does not cover all MMR genes and sample large enough to perform IHC for all the proteins. Hence IHC for MMR proteins could be performed on 75 samples only. However, both techniques can be considered to be equally proficient tests for establishing MMR/MSI status, when there is awareness of the potential pitfalls of either method [70]. Martinez-Ciarpaglini et al showed the sensitivity, specificity, positive predictive value and negative predictive value of the MMR immunostaining for the MSI status were 91%, 98%, 91% and 98%, respectively [20]. MSI status was divided into three categories: MSI-High (MSI-H), MSI-Low (MSI-L), or MS-Stable
(MSS). In general, MMR-d is considered equivalent to MSI-High21. Currently, it is recommended to classify the tumors as MSI (MMR-d) and MSS (including MSI-L and MS-stable) [71].
The frequency of MSI varies from 8.2–37% [20, 32, 48, 72, 73]. In a meta-analysis of 48 studies with a total of 18 612 patients, MSI was found in 9·2% of patients [74]. In the present study, MMR-d GC constituted 8%. The loss of expression was seen as heterodimers: loss of MLH1+PMS2 in 04/06 (66.67%) and loss of MSH2+MSH6 in 01/06 (16.67%). Isolated loss of PMS2 was seen in one (16.67%). Martinez-Ciarpaglini et al reported 18% of GC to be MMR-d by IHC which showed loss of MLH1+PMS2 in 91% and loss of MSH2+MSH6 in 9% [20]. Tsai et al in a series of 329 GC, reported PMS2/ MLH1-deficiency in12% and MSH2+MSH6 in none.10 Most MSI-GCs demonstrate loss of MLH1-expression as seen in the present study [10, 20, 64].
GC with MSI constitutes a small but relevant subgroup associated with older age, female sex, distal stomach location, and lower number of lymph-node metastases [48, 73]. In the present study, there was predominance with median age being 58 years. The tumor was predominantly located in the body in all patients. These features were in agreement with other studies [20, 48, 73] Marked to moderate lymphocytic infiltrate was seen in all the tumors. Lymph node involvement/omental nodules were present at the time of diagnosis. MSI status is reported to be associated with a better overall survival across all classifications in resectable stages of GC [48, 67, 74].
The molecular sub-typing has therapeutic implications, indicating a potential individulized treatment. The present study showed that both EBVaGC and MSI status were mutually exclusive subtypes associated with distinct clinicopathological characteristics. EBVaGC with a low mutation burden is a subset of MSS-GC and may respond to immune checkpoint therapy [20, 56]. The hypermutated nature of sporadic MSI-GC and the amplification of PD-L1 in EBVaGC make them liable for immune checkpoint blockade [74-77]. Both EBV and MSI status can be incorporated in the routine laboratory evaluation of GC, which would lead to significant progress in the treatment of gastric cancer.
Summary box: Molecular sub typing has potential benefit for individual treatment. Both EBV-associated and MSI-H-GC are considered to have better prognosis and respond to immunotherapy and hence have the possibility to be useful as biomarkers for patient stratification. EBVaGC and MMR-d GC were mutually exclusive in our study. There was no statistically significant difference in the clinicopathological variables between EBVaGC and EBVnGC as well as MMR-d GC and MMR-p GC.
Acknowledgments
Statement of Transparency and Principals:
• Author declares no conflict of interest
• Study was approved by Research Ethic Committee of author affiliated Institute.
• Study’s data is available upon a reasonable request.
• All authors have contributed to implementation of this research.
References
- Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries Sung H, Ferlay J, Siegel RL , Laversanne M, Soerjomataram I, Jemal A, Bray F. CA: a cancer journal for clinicians.2021;71(3). CrossRef
- Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): analysis of individual records for 37 513 025 patients diagnosed with one of 18 cancers from 322 population-based registries in 71 countries Allemani C, Matsuda T, Di Carlo V, Harewood R, Matz M, Nikšić M, Bonaventure A, et al . Lancet (London, England).2018;391(10125). CrossRef
- Progress in cancer survival, mortality, and incidence in seven high-income countries 1995-2014 (ICBP SURVMARK-2): a population-based study Arnold M, Rutherford MJ , Bardot A, Ferlay J, Andersson TML , Myklebust TA , Tervonen H, et al . The Lancet. Oncology.2019;20(11). CrossRef
- Epidemiology of gastric cancer: global trends, risk factors and prevention Rawla P, Barsouk A. Przegla̜d Gastroenterologiczny.2019;14(1). CrossRef
- Association between Epstein-Barr virus infection and gastric cancer: a systematic review and meta-analysis Tavakoli A, Monavari SH , Solaymani Mohammadi F, Kiani SJ , Armat S, Farahmand M. BMC cancer.2020;20(1). CrossRef
- Global burden of gastric cancer attributable to Helicobacter pylori Plummer M, Franceschi S, Vignat J, Forman D, Martel C. International Journal of Cancer.2015;136(2). CrossRef
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray F, Ferlay J, Soerjomataram I, Siegel RL , Torre LA , Jemal A. CA: a cancer journal for clinicians.2018;68(6). CrossRef
- The Two Histological Main Types Of Gastric Carcinoma: Diffuse And So-Called Intestinal-Type Carcinoma. An Attempt At A Histo-Clinical Classification Lauren P.. Acta Pathologica Et Microbiologica Scandinavica.1965;64. CrossRef
- The 2019 WHO classification of tumours of the digestive system Nagtegaal ID , Odze RD , Klimstra D, Paradis V, Rugge M, Schirmacher P, Washington KM , Carneiro F, Cree IA . Histopathology.2020;76(2). CrossRef
- An Integrative Morphomolecular Classification System of Gastric Carcinoma With Distinct Clinical Outcomes Tsai J, Jeng Y, Chen K, Lee C, Yuan C, Liau J. The American Journal of Surgical Pathology.2020;44(8). CrossRef
- Carcinoma of the stomach: A review of epidemiology, pathogenesis, molecular genetics and chemoprevention Nagini S. World Journal of Gastrointestinal Oncology.2012;4(7). CrossRef
- Comprehensive molecular characterization of gastric adenocarcinoma Cancer Genome Atlas Research Network . Nature.2014;513(7517). CrossRef
- Molecular analysis of gastric cancer identifies subtypes associated with distinct clinical outcomes Cristescu R, Lee J, Nebozhyn M, Kim K, Ting JC , Wong SS , Liu J, et al . Nature Medicine.2015;21(5). CrossRef
- Molecular Classification of Gastric Adenocarcinoma Wang Q, Liu G, Hu C. Gastroenterology Research.2019;12(6). CrossRef
- A protein and mRNA expression-based classification of gastric cancer Setia N, Agoston AT , Han HS , Mullen JT , Duda DG , Clark JW , Deshpande V, et al . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2016;29(7). CrossRef
- High-throughput Protein and mRNA Expression-based Classification of Gastric Cancers Can Identify Clinically Distinct Subtypes, Concordant With Recent Molecular Classifications Ahn S, Lee S, Kim Y, Kim A, Shin N, Choi KU , Lee C, et al . The American Journal of Surgical Pathology.2017;41(1). CrossRef
- Role of Bacterial and Viral Pathogens in Gastric Carcinogenesis Palrasu M, Zaika E, El-Rifai W, Que J, Zaika AI . Cancers.2021;13(8). CrossRef
- Helicobacter pylori and gastric cancer: Indian enigma Misra V, Pandey R, Misra SP , Dwivedi M. World Journal of Gastroenterology.2014;20(6). CrossRef
- Epstein-Barr Virus-Associated Gastric Cancer: Old Entity with New Relevance. In: Drouet, E. , editor. Epstein-Barr Virus - New Trends [Internet]. London: IntechOpen de Sousa HML , Ribeiro JPC , Timóteo MB . 2021.
- Assessing molecular subtypes of gastric cancer: microsatellite unstable and Epstein-Barr virus subtypes. Methods for detection and clinical and pathological implications Martinez-Ciarpaglini C, Fleitas-Kanonnikoff T, Gambardella V, Llorca M, Mongort C, Mengual R, Nieto G, et al . ESMO open.2019;4(3). CrossRef
- A National Cancer Institute Workshop on Microsatellite Instability for cancer detection and familial predisposition: development of international criteria for the determination of microsatellite instability in colorectal cancer Boland C. R., Thibodeau S. N., Hamilton S. R., Sidransky D., Eshleman J. R., Burt R. W., Meltzer S. J., et al . Cancer Research.1998;58(22). CrossRef
- Clinical Significance of Four Molecular Subtypes of Gastric Cancer Identified by The Cancer Genome Atlas Project Sohn BH , Hwang J, Jang H, Lee H, Oh S, Shim J, Lee K, et al . Clinical Cancer Research: An Official Journal of the American Association for Cancer Research.2017;23(15). CrossRef
- Profiling cancer-associated genetic alterations and molecular classification of cancer in Korean gastric cancer patients Kim Y, Cho M, Kim J, Kim SN , Oh SC , Lee K. Oncotarget.2017;8(41). CrossRef
- Gastric cancer in India Sharma A, Radhakrishnan V. Indian Journal of Medical and Paediatric Oncology: Official Journal of Indian Society of Medical & Paediatric Oncology.2011;32(1). CrossRef
- Gastric cancer-a clinicopathological study in a tertiary care centre of North-eastern India Barad AK , Mandal SK , Harsha HS , Sharma BM , Singh TS . Journal of Gastrointestinal Oncology.2014;5(2). CrossRef
- Epstein-Barr virus-associated gastric carcinoma in southern India: A comparison with a large-scale Japanese series Kattoor J, Koriyama C, Akiba S, Itoh T, Ding S, Eizuru Y, Abraham EK , Chandralekha B., Amma N. S., Nair MK . Journal of Medical Virology.2002;68(3). CrossRef
- Burden of Gastric Cancer Thrift AP , El-Serag HB . Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association.2020;18(3). CrossRef
- The current and future incidence and mortality of gastric cancer in 185 countries, 2020-40: A population-based modelling study Morgan E, Arnold M, Camargo MC , Gini A, Kunzmann AT , Matsuda T, Meheus F, et al . EClinicalMedicine.2022;47. CrossRef
- A human model of gastric carcinogenesis Correa P.. Cancer Research.1988;48(13). CrossRef
- Diet, H pylori infection and gastric cancer: evidence and controversies Rocco A, Nardone G. World Journal of Gastroenterology.2007;13(21). CrossRef
- Causal role of Helicobacter pylori infection in gastric cancer: an Asian enigma Singh K, Ghoshal UC . World Journal of Gastroenterology.2006;12(9). CrossRef
- Epstein-Barr virus-positive gastric cancer: a distinct molecular subtype of the disease? Jácome AADA , Lima EM , Kazzi AI , Chaves GF , Mendonça DC , Maciel MM , Santos JSD . Revista Da Sociedade Brasileira De Medicina Tropical.2016;49(2). CrossRef
- Thirty years of Epstein-Barr virus-associated gastric carcinoma Fukayama M, Abe H, Kunita A, Shinozaki-Ushiku A, Matsusaka K, Ushiku T, Kaneda A. Virchows Archiv: An International Journal of Pathology.2020;476(3). CrossRef
- Epstein-Barr virus in gastric carcinoma Tokunaga M., Land C. E., Uemura Y., Tokudome T., Tanaka S., Sato E.. The American Journal of Pathology.1993;143(5).
- Epstein-Barr virus and gastric carcinoma Fukayama M. Pathology International.2010;60(5). CrossRef
- Update on Epstein-Barr virus and gastric cancer (review) Shinozaki-Ushiku A, Kunita A, Fukayama M. International Journal of Oncology.2015;46(4). CrossRef
- Clinical Importance of Epstein⁻Barr Virus-Associated Gastric Cancer Nishikawa J, Iizasa H, Yoshiyama H, Shimokuri K, Kobayashi Y, Sasaki S, Nakamura M, et al . Cancers.2018;10(6). CrossRef
- Epstein-Barr virus and gastric carcinoma Takada K.. Molecular pathology: MP.2000;53(5). CrossRef
- Epstein-Barr virus-associated gastric cancer reveals intratumoral heterogeneity of PIK3CA mutations Böger C., Krüger S., Behrens H. M., Bock S., Haag J., Kalthoff H., Röcken C.. Annals of Oncology: Official Journal of the European Society for Medical Oncology.2017;28(5). CrossRef
- EBV-positive gastric adenocarcinomas: a distinct clinicopathologic entity with a low frequency of lymph node involvement Beek J, Hausen A, Klein Kranenbarg E, Velde CJH , Middeldorp JM , Brule AJC , Meijer CJLM , Bloemena E. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2004;22(4). CrossRef
- Low prevalence of Epstein-Barr virus in incident gastric adenocarcinomas from the United Kingdom Burgess D. E., Woodman C. B., Flavell K. J., Rowlands D. C., Crocker J., Scott K., Biddulph J. P., Young L. S., Murray P. G.. British Journal of Cancer.2002;86(5). CrossRef
- Epstein-Barr virus-associated gastric carcinoma: Evidence of age-dependence among a Mexican population Herrera-Goepfert R, Akiba S, Koriyama C, Ding S, Reyes E, Itoh T, Minakami Y, Eizuru Y. World Journal of Gastroenterology.2005;11(39). CrossRef
- Prevalence and characteristics of Epstein-Barr virus-associated gastric cancer in Iran Faghihloo E, Saremi MR , Mahabadi M, Akbari H, Saberfar E. Archives of Iranian Medicine.2014;17(11).
- Prevalence and characteristics of Epstein-Barr virus-associated gastric carcinomas in Tunisia Trimeche M, Ksiâa F, Ziadi S, Mestiri S, Hachana M, Gacem RB , Sriha B, Korbi S. European Journal of Gastroenterology & Hepatology.2009;21(9). CrossRef
- Meta-analysis shows that prevalence of Epstein-Barr virus-positive gastric cancer differs based on sex and anatomic location Murphy G, Pfeiffer R, Camargo MC , Rabkin CS . Gastroenterology.2009;137(3). CrossRef
- Epstein-Barr virus-associated gastric adenocarcinoma Shibata D., Weiss L. M.. The American Journal of Pathology.1992;140(4). CrossRef
- Validation and calibration of next-generation sequencing to identify Epstein-Barr virus-positive gastric cancer in The Cancer Genome Atlas Camargo MC , Bowlby R, Chu A, Pedamallu CS , Thorsson V, Elmore S, Mungall AJ , Bass AJ , Gulley ML , Rabkin CS . Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association.2016;19(2). CrossRef
- Reproduction of the Cancer Genome Atlas (TCGA) and Asian Cancer Research Group (ACRG) Gastric Cancer Molecular Classifications and Their Association with Clinicopathological Characteristics and Overall Survival in Moroccan Patients Nshizirungu JP , Bennis S, Mellouki I, Sekal M, Benajah D, Lahmidani N, El Bouhaddouti H, et al . Disease Markers.2021;2021. CrossRef
- Microsatellite Instability: A Predictive Biomarker for Cancer Immunotherapy Chang L, Chang M, Chang HM , Chang F. Applied immunohistochemistry & molecular morphology: AIMM.2018;26(2). CrossRef
- Lymphocyte-rich gastric cancer: associations with Epstein-Barr virus, microsatellite instability, histology, and survival Grogg KL , Lohse CM , Pankratz VS , Halling KC , Smyrk TC . Modern Pathology: An Official Journal of the United States and Canadian Academy of Pathology, Inc.2003;16(7). CrossRef
- A combined histologic and molecular approach identifies three groups of gastric cancer with different prognosis Solcia E, Klersy C, Mastracci L, Alberizzi P, Candusso ME , Diegoli M, Tava F, et al . Virchows Archiv: An International Journal of Pathology.2009;455(3). CrossRef
- Epstein-Barr virus positivity, not mismatch repair-deficiency, is a favorable risk factor for lymph node metastasis in submucosa-invasive early gastric cancer Park JH , Kim EK , Kim YH , Kim J, Bae YS , Lee YC , Cheong J, Noh SH , Kim H. Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association.2016;19(4). CrossRef
- Epstein-Barr virus DNA load and its association with Helicobacter pylori infection in gastroduodenal diseases Shukla SK , Prasad K. N., Tripathi A, Singh A, Saxena A, Ghoshal UC , Krishnani N, Husain N. The Brazilian Journal of Infectious Diseases: An Official Publication of the Brazilian Society of Infectious Diseases.2011;15(6).
- Epstein-Barr Virus-Associated Gastric Carcinoma: Use of Host Cell Machineries and Somatic Gene Mutations Abe H, Kaneda A, Fukayama M. Pathobiology: Journal of Immunopathology, Molecular and Cellular Biology.2015;82(5). CrossRef
- Potential prognostic impact of EBV RNA-seq reads in gastric cancer: a reanalysis of The Cancer Genome Atlas cohort Sadato D, Ogawa M, Hirama C, Hishima T, Horiguchi S, Harada Y, Shimoyama T, et al . FEBS open bio.2020;10(3). CrossRef
- EBV-Positive Gastric Cancer: Current Knowledge and Future Perspectives Sun K, Jia K, Lv H, Wang S, Wu Y, Lei H, Chen X. Frontiers in Oncology.2020;10. CrossRef
- Features of Gastric Carcinoma With Lymphoid Stroma Associated With Epstein-Barr Virus Lim H, Park YS , Lee JH , Son DH , Ahn JY , Choi K, Kim DH , et al . Clinical Gastroenterology and Hepatology: The Official Clinical Practice Journal of the American Gastroenterological Association.2015;13(10). CrossRef
- Clinicopathological and molecular characteristics of Epstein-Barr virus-associated gastric carcinoma: a meta-analysis Lee J, Kim S, Han S, An J, Lee E, Kim Y. Journal of Gastroenterology and Hepatology.2009;24(3). CrossRef
- Epstein-Barr virus infection serves as an independent predictor of survival in patients with lymphoepithelioma-like gastric carcinoma Min B, Tae CH , Ahn SM , Kang SY , Woo S, Kim S, Kim K. Gastric Cancer: Official Journal of the International Gastric Cancer Association and the Japanese Gastric Cancer Association.2016;19(3). CrossRef
- Epstein-Barr virus-associated gastric carcinoma and atrophic gastritis Yanai H., Murakami T., Yoshiyama H., Takeuchi H., Nishikawa J., Nakamura H., Okita K., et al . Journal of Clinical Gastroenterology.1999;29(1). CrossRef
- Evaluation of epstein-barr virus DNA load in gastric mucosa with chronic atrophic gastritis using a real-time quantitative PCR assay Hirano A, Yanai H, Shimizu N, Okamoto T, Matsubara Y, Yamamoto K, Okita K. International Journal of Gastrointestinal Cancer.2003;34(2-3). CrossRef
- Recent patterns in gastric cancer: a global overview Bertuccio P, Chatenoud L, Levi F, Praud D, Ferlay J, Negri E, Malvezzi M, La Vecchia C. International Journal of Cancer.2009;125(3). CrossRef
- Three Molecular Subtypes of Gastric Adenocarcinoma Have Distinct Histochemical Features Reflecting Epstein-Barr Virus Infection Status and Neuroendocrine Differentiation Speck O, Tang W, Morgan DR , Kuan PF , Meyers MO , Dominguez RL , Martinez E, Gulley ML . Applied immunohistochemistry & molecular morphology: AIMM.2015;23(9). CrossRef
- Predictive biomarkers in gastric cancer Röcken C.. Journal of Cancer Research and Clinical Oncology.2023;149(1). CrossRef
- Abundant PD-L1 expression in Epstein-Barr Virus-infected gastric cancers Derks S, Liao X, Chiaravalli AM , Xu X, Camargo MC , Solcia E, Sessa F, et al . Oncotarget.2016;7(22). CrossRef
- The Predictive Value of Epstein-Barr Virus-Positivity in Patients Undergoing Gastrectomy Followed by Adjuvant Chemotherapy Baek DW , Kang BW , Kim JG . Chonnam Medical Journal.2018;54(3). CrossRef
- Microsatellite instability in Gastric Cancer: Between lights and shadows Puliga E, Corso S, Pietrantonio F, Giordano S. Cancer Treatment Reviews.2021;95. CrossRef
- Comparison of microsatellite instability detection by immunohistochemistry and molecular techniques in colorectal and endometrial cancer Dedeurwaerdere F, Claes KB , Van Dorpe J, Rottiers I, Van der Meulen J, Breyne J, Swaerts K, Martens G. Scientific Reports.2021;11(1). CrossRef
- Microsatellite instability: a review of what the oncologist should know Li K, Luo H, Huang L, Luo H, Zhu X. Cancer Cell International.2020;20. CrossRef
- Identifying mismatch repair-deficient colon cancer: near-perfect concordance between immunohistochemistry and microsatellite instability testing in a large, population-based series Loughrey MB , McGrath J, Coleman HG , Bankhead P, Maxwell P, McGready C, Bingham V, et al . Histopathology.2021;78(3). CrossRef
- Sensitive detection of microsatellite instability in tissues and liquid biopsies: Recent developments and updates Yu F, Makrigiorgos A, Leong KW , Makrigiorgos GM . Computational and Structural Biotechnology Journal.2021;19. CrossRef
- Neoadjuvant therapy strategies for advanced gastric cancer: Current innovations and future challenges Zhu Z, Gong Y, Xu H. Chronic Diseases and Translational Medicine.2020;6(3). CrossRef
- Microsatellite instability in gastric cancer: molecular bases, clinical perspectives, and new treatment approaches Ratti M, Lampis A, Hahne JC , Passalacqua R, Valeri N. Cellular and molecular life sciences: CMLS.2018;75(22). CrossRef
- Meta-analysis of microsatellite instability in relation to clinicopathological characteristics and overall survival in gastric cancer Polom K., Marano L., Marrelli D., De Luca R., Roviello G., Savelli V., Tan P., Roviello F.. The British Journal of Surgery.2018;105(3). CrossRef
- Current Status of Immune Checkpoint Inhibitors in Gastrointestinal Cancers Kim BJ , Jang HJ , Kim HS , Kim JH . Journal of Cancer.2017;8(8). CrossRef
- Chemosensitivity and survival in gastric cancer patients with microsatellite instability Oki E, Kakeji Y, Zhao Y, Yoshida R, Ando K, Masuda T, Ohgaki K, Morita M, Maehara Y. Annals of Surgical Oncology.2009;16(9). CrossRef
- Microsatellite instability in sporadic gastric cancer: its prognostic role and guidance for 5-FU based chemotherapy after R0 resection An JY J, Kim H, Cheong J, Hyung WJ , Kim H, Noh SH . International Journal of Cancer.2012;131(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times