Dosimetric Comparison with Different ARC and Beam VMAT Optimization in the Monaco Treatment Planning System
Download
Abstract
Introduction: The Monaco Treatment planning system provides the sequencing parameter ‘Max Number of Arcs’ in volumetric modulated arc therapy optimization. Using this, we can have a maximum of four arcs per beam. In this study, different arc and beam combinations are evaluated.
Aim: To compare the dosimetric parameters of VMAT plans in oral cavity radiotherapy treatment planning using single beam multiple arcs and two beam single arcs, and thereby evaluate the effects of the ‘Max Number of Arcs’ optimization parameter in the Monaco treatment planning system.
Materials and methods: Data from 10 previously treated head and neck cancer (oral cavity) patients were used for this study. For each patient, four plans were generated with different arc and beam arrangements (one beam with 4 arcs, one beam with 3 arcs, one beam with 2 arcs, and two beams with a single arc). Plans were generated with similar constraints and dose coverage objectives, and all plans were normalized to receive 95% of the PTV receiving 100% of the prescription dose. Plans were evaluated using different dosimetric parameters.
Results and discussion: Using one beam with four arc optimization parameter, homogeneous plans were generated, compromising delivery time and MU. One beam three arc plan gives comparative dose coverage, reduced delivery time, and MU compared to one beam four arc plan. One beam with two arc shows poor coverage. Two beam, one arc doesn’t give the expected result with a higher Dmax value, but MU and delivery times are on par with the three beam, one arc plan.
Conclusion: For complex head and neck plans, one beam with three arcs is recommended for obtaining the optimum dose coverage, delivery time, and MU. This leads to reduced patient-on-table time and quality assurance time.
Introduction
Radiotherapy is an integral part of most oral cavity treatments and is an important part of the overall management of many of these tumours. The main aim of using radiotherapy in head and neck cancers is higher dose delivery with organ sparing [1]. With modern techniques, we can achieve the goal of radiotherapy with more accuracy. Volumetric-modulated arc therapy (VMAT) can cover the entire planning target volume (PTV) from single or multiple arcs. During VMAT delivery, the intensity of the beam is modulated by varying the gantry rotation speed, multileaf collimator (MLC) shape and dose rate [2, 3]. The beam is ON during the entire treatment while the gantry rotates around the patient. VMAT is superior in sparing the critical organs, reducing delivery time, achieving target coverage, and reducing the dose to organs at risk (OARs) in a variety of clinical studies [4-7] when compared to other radiotherapy techniques like intensity-modulated radiation therapy (IMRT) and 3-dimensional conformal therapy techniques.
Usually selection of arcs depends on the complexity of the target volume considering the organs at risk. Especially for head and neck contours, which includes complex planning. Also, the selection of arc is different in different planning systems. Monaco (version 5.2) treatment planning system has a subarc (maximum 4 arcs) selection option using the “max arcs-per-beam” optimization parameter. This parameter helps to deliver multiple arcs using a single collimator angle and the gantry rotates clockwise and counterclockwise in a continuous beam ON state, which gives more freedom for the optimization algorithm in optimizing. This is different from the Eclipse planning system’s multiple arcs. Usually increasing the number of arcs increases the delivery time, because, each VMAT arc delivery and recording involves time. Even though multiple arcs and single arc studies are conducted using different planning systems, the “max arcs-per-beam” optimization parameter is used in limited studies and as per our knowledge no studies were found using VMAT plans for head and neck sites. Studies [8] had been conducted on the use of multiple arcs which resulted in better target coverage and dose homogeneity but compromised on larger low dose, more monitor units (MU), and longer delivery times. Also found that two-arc plans are better in dose coverage and conformity but again compromise on delivery time compared to single-arc plans [7, 10]. Generally, number of arcs is selected considering the complexity of the target volume. In the case of the Monaco planning system, a multiple-arc single beam is a beam that has at least 2 arc segments, where one segment rotates clockwise and the other rotates counterclockwise [9, 10]. To know about the different arc selection options available with the Monaco planning system, different beam and arc combinations were checked in this study. Hence the dosimetric difference between the single beam-multi arc and 2 beam-single arcs VMAT plans in terms of treatment delivery time, and other dosimetric parameters were evaluated also to find the impact of the same on the clinical workflow of Head and neck cases
Materials and Methods
Patient Selection
To construct dose matrices for methodological comparison, 10 previously treated patients with various stages of cancer of the oral cavity after undergoing surgery in our institute were included in this study. Patients were treated in Elekta Versa HD Machine (Elekta Oncology Systems, Crawley, UK).
Target Volume delineation
Individualized thermoplastic immobilization were prepared for all patients and underwent planning CT with contrast with a slice thickness of 2.5mm. The target volume delineation was done as per the institutional protocol [11]. High risk clinical target volume (HR CTV) included tumour bed with 1 cm margin and any lymph node levels with pathologically proven positive lymph nodes. For carcinoma tongue, entire tongue was included in the high risk volume. Low risk clinical target volume (LR CTV) included electively treated neck node levels in addition to the high risk volume.
Organs at risk (OARs) delineated for all patients included the spinal cord, the brain stem, both parotid glands, the larynx, the cervical oesophagus, pharyngeal constrictors, middle ear, the inner ear, the mandible and oral cavity. The spinal cord and brain stem contours were expanded by 5mm to generate the respective planning OAR volumes.
Radiation Therapy Planning Techniques
Dosimetric data of already treated carcinoma oral cavity cases were retrieved from the Monaco treatment planning system (version 5.2) Two planning target volumes (PTVs) were defined: PTV high risk (PTV HD) obtained by expanding the HR CTV by 5mm and PTV low risk (PTV LD) obtained by expanding the LR CTV by 5mm. The prescription dose was 60Gy for high dose volume (PTV HD) and 54Gy for low dose volume (PTV LD). For each patient, 4 plans were generated using coplanar VMAT in the Monaco TPS using the Monte Carlo algorithm. 3 plans with single beam multiple arcs (one beam with 4 arcs, one beam with 3 arcs and one beam with 2 arcs) and one plan with two beams with one arc were performed. All constraints and dose coverage were kept the same between plans, and all plans were normalized to ensure 95% of the PTV received 100% of the prescription dose. The sequential parameter window in the Monaco Treatment planning system, where we can change the number of arcs is displayed in Figure 1.
Figure 1. Sequencing Parameter Window of Monaco Planning System.
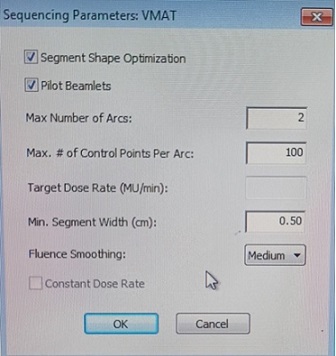
Plans were generated changing the maximum number of arcs and beam number. Organ dose constraints followed the QUANTEC recommendations [12]. The planning objectives used in VMAT planning are given in Table 1.
| Structures | Planning Objectives |
| PTV60 | V60 Gy≥95% |
| PTV54 | V54 Gy≥95% |
| Spinal Cord | Max 45Gy |
| Parotid | D50% < 30 Gy, Dmean< 26 Gy |
| Esophagus | V95Gy<33% |
| Brainstem | Entire brainstem<54 Gy,V59Gy<1-10CC |
| Eye | Mean<35Gy,Max 54Gy |
| Lens | Max7Gy |
| Chiasm/Optic Nerves | Max 55Gy |
| Mandible | Max 70Gy,V75<1CC |
| Normal Tissues | As low as possible |
PTV, planning target volume;VxGy, volume receiving at least x-Gy dose
Treatment Plan Evaluation
Single-beam multiple arcs and two beam Single arc plans were quantitatively compared in terms of planning target volume (PTV) coverage, conformity index, homogeneity index, treatment delivery time and number of monitor units.
Homogeneity Index (HI), Conformity Index (CI) are calculated using the following formulas,
Homogeneity Index (HI) = (D2%-D98%)/D50% - (1) where D2%, D98% and D50% are dose to 2%, 98% and 50% volumes of the PTV.
Conformity Index (CI) = (TVPI)² / (PI x TV) - (2)
Where PI is the volume of tissue covered by the prescription isodoseline (95%). TVPI is the volume of target covered by the prescription isodose line (95%) and TV is the total target volume.
Quality Assurance
To check the delivery time of each plan, quality assurance was performed by calculating QA plans for all the forty plans created for ten oral cavity cases. The plans were exported to Elekta versa HD linac with Agility collimator, and delivered to Octavius QA phantom with 2D Array detector (PTW, Germany) under standard quality assurance (QA) conditions using gamma (γ) value of %/3 mm. During the QA plan delivery to Phantom, we recorded the delivery time for each plan and the mean value is tabulated in Table 2.
| Plan | MU | CI | HI | Delivery Time | Dmax |
| Plan 1 | 1013.80±79.94 | 0.900±0.00 | 0.09±0.00 | 4.02±0.16 | 6484.54±327.64 |
| Plan2 | 944.69±94.59 | 0.995±0.1042 | 0.08±0.01 | 3.33±0.15 | 6632.45±147.16 |
| Plan3 | 862.69±91.17 | 0.91±0.01 | 0.09±0.01 | 2.36±0.54 | 6699.92±156.35 |
| Plan4 | 954.57±112.35 | 0.95±0.0.11 | 0.27±0.24 | 3.28±0.18 | 7351.68±523.49 |
| P Value | 0 | 0.418 | 0.489 | 0 | 0 |
Values ± standard deviation. CI, conformity index; HI, homogeneity index; MU, monitor units; Dmax, Maximum dose. Plan1- One beam 4arc; Plan2 – One beam 3arc; Plan3- One beam 2 arc; Plan 4- Two beam one arc
A passing rate of above 95% was achieved for all the plans.
Statistical Analysis
Data were analysed using SPSS statistical software, version 20.0. Descriptive and inferential statistical methods were used for analyzing the study results. One- way analysis of variance (ANOVA) is used to find out the relation between the variables
Results
Demographic Profile
Ten patients (eight males and two females) were enrolled in the study, with age ranging from 45 to 67 years. Primary tongue cases were selected for the study.
Treatment Details
The mean difference between factors associated with Plan quality is tabulated in Table 3.
| PTV | Mean Volume | Treatment Plan | D2 | D98 | D50 |
| plan1 | 6325±29.94 | 5944.70±20.89 | 6177.19±24.34 | ||
| PTV HD | plan2 | 6464.33±50.30 | 5899.45±84.64 | 6178.86±47.07 | |
| 246.45±48.91 | plan3 | 6487.19±66.70 | 5905.06±17.85 | 6274.34±48.53 | |
| plan4 | 6971.67±470.96 | 5267.06±1658.57 | 6452.81±298.88 | ||
| P Value | 0.468 | 0.216 | 0 | ||
| plan1 | 6284.54±48.05 | 5383.63±64.29 | 5810.35±125.93 | ||
| PTV LD | 589.18±111.73 | plan2 | 6317.63±56.62 | 5405.19±100.08 | 5870.03±128 |
| plan3 | 6429.49±88.27 | 5354.71±77.74 | 5933.23±116.57 | ||
| plan4 | 6880.11±428.56 | 5047.57±187.26 | 6106.12±275.09 | ||
| P Value | 0 | 0 | 0 |
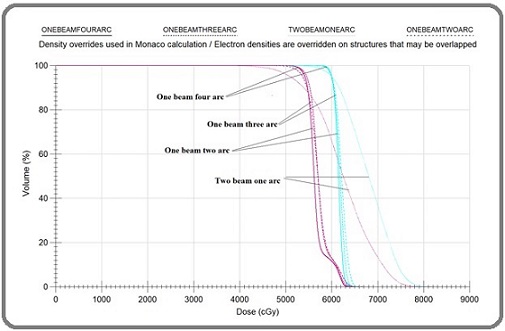
Treatment plans with one beam four arcs, one beam three arcs, one beam two arcs and two beams one arc are represented as plan1, plan2, plan3 and plan4 respectively. No significant differences were observed between the D2 and D98 values of PTV HD. However, significant variation is observed in the D50 values across the four plans. D2, D98 and D50 values of PTV LD are significant compared to PTV HD Table 2 gives the conformity index (CI), homogeneity index (HI), monitor units (MU), Maximum dose (Dmax) and treatment delivery time of different plans; Plan1- One beam with 4arcs; Plan2 – One beam with 3arcs; Plan3- One beam with 2 arcs; Plan 4- Two beam with one arc. Among these significant differences were observed for MU values, Dmax and delivery time. A maximum delivery time of 4.02±0.16 minutes for one beam four arc and a minimum delivery time of 2.36±0.54 for one beam two arc plan. For one beam three arc and two beam one arc plans delivery times are almost equal with 3.33±0.15 and 3.28±0.18 respectively. The dose distribution and dose-volume histogram of the four distinct plans are illustrated in Figures 2 and 3, respectively.
Figure 2. Representation of PTV Coverage for a Single Case. A) One beam three Arc; B) One beam four Arc; C) Two Beam single Arc; D) One beam two arc.
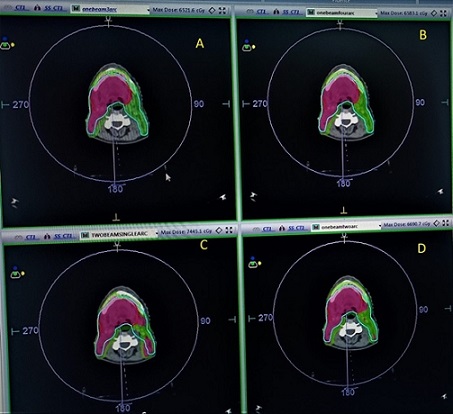
Figure 3. Represents the Dose Volume Histograms of PTV LD and PTV HD of All the Four Plans.
Discussion
Benefit of varying arc beam combinations is the potential to reduce treatment time. But the specific choice of arc combinations can further optimize treatment time. Shorter treatment durations can reduce the cumulative radiation exposure to healthy tissues over the course of the treatment, thereby lowering the risk of late-onset side effects such as organ dysfunction or secondary cancers [13]. Homogeneous dose distribution can reduce the risk of over-irradiating certain areas, which can cause acute side effects such as nausea, vomiting, or skin irritation. For instance, in head and neck cancer treatments, careful manipulation of arc combinations can limit the radiation dose to the salivary glands, potentially reducing xerostomia (dry mouth), a common and debilitating side effect [14]. Using multiple arcs allows for dose distribution from different angles, which can help reduce the overall dose to critical structures such as the spinal cord, lungs, or heart. By optimizing the arc combinations, the Monaco system allows for the fine-tuning of dose gradients around the tumor, balancing the need for high- dose delivery to the target with the goal of minimizing dose to surrounding healthy tissues.
No significant differences were observed between the D2 and D98 values of PTV HD (Table 3). However, significant variation is observed in the D50 values across the four plans. D2, D98 and D50 values of PTV LD are significant compared to PTV HD. This may be due to the comparably larger volume of PTV LD (589.18±111.73) compared to PTV HD (246.45 ± 46.86). MU and delivery time are higher for one beam with four arc plans, even though it has a good and homogeneous coverage with less dose spill and Dmax value. This is in accordance with the literature review, which indicates that multiple-arc VMAT improves results compared to single-arc VMAT, at the cost of increased delivery times, higher monitor units, and a greater spread of low doses [8].
Another study compared single arc, double arc and IMRT plans for prostate VMAT and concluded that the double arc plan achieved the best dosimetric quality with the highest minimum PTV dose, lowest hotspot, and the best homogeneity and conformity compared to IMRT and single arc plans [10]. But in our study, two beam one arc plan has less delivery time but has a higher Dmax thereby delivering a non-homogeneous plan. One beam three arc plan delivered a reasonably good plan in almost all oral cavity plans with optimum MU, delivery time and Dmax. The dose distribution of one beam with three arc plan is comparable with that of one beam with four arc plans. One beam three arc and two beam one arc has almost similar delivery time. Like our study, Alan M. Kalet found that 2 APB planning has a dosimetric advantage over 1 APB planning for complex PTV volumes and for all 2 APB plans, a reduction in QA time, QA effort and clinical delivery time is achieved without loss to dosimetric plan quality [15, 16].
It is also observed that the Dmax dose is reduced when we employ more number of arc (subarc). In the Monaco planning system, the available maximum number of arcs (sub-arc) is four. Our study shows good coverage and OAR sparing with a higher number of sub-arcs, but it results in higher MU and delivery time, making it difficult to manage in a busy department.
Dose distribution is best for plans using one beam multiple arcs compared to two beam one arc. Delivery time of one beam three arc and two beam one arc are comparable. Also, with lower Dmax, better homogeneity and conformity compared to two beam one arc plans. Hence one beam of three arcs will be an optimum choice for complex head and neck plans for the Monaco planning system.
Small sample size is a limitation of this study. For ten- sample size, 40 plans (4 plans per patient) were generated for the study. Further research with larger sample sizes will help to confirm and extend these findings.
In conclusion, in this study, single beam with multiple arc and two beam single arc VMAT plans were compared for Monaco TPS. One beam two arc plans have the least delivery time and Dmax but failed to give homogeneous distribution compared to one beam three arc and one beam four arc plans. The Two beam single arc plan has a reasonable delivery time but shows a higher Dmax value when compared with the other three plans. Monaco TPS gives good plans for the increased number of arcs but compromises delivery time. One beam with three arc gives an optimum result compared to other plans.
Acknowledgments
Statement of Transparency and Principals
• Author declares no conflict of interest
• Study was approved by Research Ethic Committee
of author affiliated Institute.
• Study’s data is available upon a reasonable request.
• All authors have contributed to implementation of this research.
References
- Concomitant chemoradiotherapy as primary therapy for locoregionally advanced head and neck cancer Vokes E. E., Kies M. S., Haraf D. J., Stenson K., List M., Humerickhouse R., et al . Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2000;18(8). CrossRef
- Commissioning and quality assurance of RapidArc radiotherapy delivery system Ling CC , Zhang P, Archambault Y, Bocanek J, Tang G, Losasso T. International Journal of Radiation Oncology, Biology, Physics.2008;72(2). CrossRef
- Dosimetric verification of RapidArc treatment delivery Korreman S, Medin J, Kjaer-Kristoffersen F. Acta Oncologica (Stockholm, Sweden).2009;48(2). CrossRef
- A treatment planning study comparing volumetric arc modulation with RapidArc and fixed field IMRT for cervix uteri radiotherapy Cozzi L, Dinshaw KA , Shrivastava SK , Mahantshetty U, Engineer R, Deshpande DD , Jamema S. V., et al . Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2008;89(2). CrossRef
- Treatment planning for volumetric modulated arc therapy Bedford JL . Medical Physics.2009;36(11). CrossRef
- Development and evaluation of an efficient approach to volumetric arc therapy planning Bzdusek K, Friberger H, Eriksson K, Hårdemark B, Robinson D, Kaus M. Medical Physics.2009;36(6). CrossRef
- Intensity-modulated arc therapy: principles, technologies and clinical implementation Yu CX , Tang G. Physics in Medicine and Biology.2011;56(5). CrossRef
- Is a single arc sufficient in volumetric-modulated arc therapy (VMAT) for complex-shaped target volumes? Guckenberger M, Richter A, Krieger T, Wilbert J, Baier K, Flentje M. Radiotherapy and Oncology: Journal of the European Society for Therapeutic Radiology and Oncology.2009;93(2). CrossRef
- RapidArc radiotherapy planning for prostate cancer: single-arc and double-arc techniques vs. intensity-modulated radiotherapy Sze HCK , Lee MCH , Hung W, Yau T, Lee AWM . Medical Dosimetry: Official Journal of the American Association of Medical Dosimetrists.2012;37(1). CrossRef
- Dual arc volumetric-modulated arc radiotherapy (VMAT) of nasopharyngeal carcinomas: a simultaneous integrated boost treatment plan comparison with intensity-modulated radiotherapies and single arc VMAT Lee T.-F., Ting H.-M., Chao P.-J., Fang F.-M.. Clinical Oncology (Royal College of Radiologists (Great Britain)).2012;24(3). CrossRef
- Locoregional recurrences after post-operative volumetric modulated arc radiotherapy (VMAT) in oral cavity cancers in a resource constrained setting: experience and lessons learned Chakraborty S., Patil V. M., Babu S., Muttath G., Thiagarajan S. K.. The British Journal of Radiology.2015;88(1048). CrossRef
- Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues Bentzen SM , Constine LS , Deasy JO , Eisbruch A, Jackson A, Marks LB , Ten Haken RK , Yorke ED . International Journal of Radiation Oncology, Biology, Physics.2010;76(3 Suppl). CrossRef
- Radiation therapy-associated toxicity: Etiology, management, and prevention Wang K, Tepper JE . CA: a cancer journal for clinicians.2021;71(5). CrossRef
- Radiotherapy dose-volume effects on salivary gland function Deasy JO , Moiseenko V, Marks L, Chao KSC , Nam J, Eisbruch A. International Journal of Radiation Oncology, Biology, Physics.2010;76(3 Suppl). CrossRef
- SU-E-T-384: Combining Multiple Arcs Into a Single Arc for Efficient Delivery Oddiraju S, Rangaraj D, Papiez L. Medical Physics.2013;40(6Part16). CrossRef
- Dosimetric comparison of single-beam multi-arc and 2-beam multi-arc VMAT optimization in the Monaco treatment planning system Kalet AM , Richardson HL , Nikolaisen DA , Cao N, Lavilla MA , Dempsey C, Meyer J, Koh W, Russell KJ . Medical Dosimetry: Official Journal of the American Association of Medical Dosimetrists.2017;42(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2025
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times