Correlation of Hematological Toxicity with the Bone Marrow Radiation Dose and Volume during Concurrent Chemo Radiation in Patients with Cervical Cancer
Download
Abstract
Aims & Objective: Hematological toxicity is common in patients with cervical cancer treated with concurrent chemo radiotherapy (CT-RT), so the purpose is to assess this hematological toxicity and correlate the toxicity with the dose and volume of bone marrow included in the field of radiation.
Materials & Methods: Twenty five patients with histologically proven cervical cancer attending to our Cancer centre from July 2018-August 2019 were the subjects of this study. Patients were treated on 6 MV linear accelerator with a radical intent with concurrent chemotherapy using cisplatin 50 mg weekly. The planning CT was done for all the patients before the treatment and contouring of the pelvic bone marrow apart from other organs at risk was done. Hematological toxicity was assessed using RTOG common toxicity criteria weekly during and at 2 weeks after the completion of the treatment.
Results: A total of 25 patients on CT-RT treatment were assessed. Sixteen patients were in locally advanced stage. The variation in HB, TLC, Platelets, and ANC counts from the baseline to 2 weeks after chemo radiotherapy were assessed. Grade II anemia was observed in 12 and Grade III in 2 patients. There were no toxicity as far as WBC and platelets were considered. There was also no correlation between the volume of bone marrow included in the field of irradiation and appearance of anemia.
Conclusion: CT-RT for cervical cancer is safe and is associated with minimal hematological toxicity in the form of anemia. The toxicity is same for different volumes of bone marrow included in the field of irradiation with both 3DCRT as well as IMRT technique. The toxicity observed is probably contributed by Cisplatin.
Introduction
Cervical cancer is the second most common cancer worldwide among women next to breast cancer and is the primary cause of cancer related deaths in developing countries. Each year cervical cancer is diagnosed in about 5,00,000 women globally and is responsible for 2,60,000 deaths annually [1].
Approximately one fourth of the world cases of cervical cancer detected each year in India and the highest incidence is seen in Chennai, lowest in Delhi and the incidence in Bangalore is 21.7/lakh. In our department, cervical cancer formed 18.39% of total cases of cancers from 1998-2005 [2].
Most of the patients are diagnosed in locally advanced stage for which concurrent chemo radiation is the treatment of choice which is followed by brachytherapy. Concurrent chemotherapy and radiation became the standard of care for cervical cancer since 1999-2000. National cancer institute made a clinical announcement stating that cisplatin based chemotherapy is the new standard of therapy for cervical cancer. This NCI alert came on 23 February, 1999 showed that platinum based chemotherapy used along with radiation had twelve percent increase in local control/survival [3].
Cisplatin chemotherapy is known for its severe hematological toxicity. Pelvic radiation adds to this and is related to the extent of bone marrow that is involved in the field of radiation. The combined toxicity when severe, leads to interruption in treatment and any delay in completion of planned treatment is associated with a reduced probability of local control in patients receiving curative treatment. Several studies have suggested that there may be as much as 1% decrease in survival and local control for each extra day of treatment beyond a total treatment time of 55-60 days [4].
The hematological toxicity is assessed weekly once throughout the course of radiation and grading of toxicity is done as per RTOG toxicity criteria version 2. With the availability of CT scan for simulation, tumor as well as critical structures is delineated and the dose received by them can be documented. The volume and dose of bone marrow could be correlated with the weekly blood picture values for a better understanding. The pattern of hematological toxicity would probably help us in framing new guidelines for their prevention and early intervention. There are many studies available with sophisticated technology like IMRT with pelvic irradiation; however there is a paucity of literature with conventional techniques as well as 3DCRT. Hence the need for this study.
Aims and Objectives
1. To assess the hematological toxicity during concurrent chemo radiation in patients with cervical cancer.
2. To correlate hematological toxicity with the dose and
volume of bone marrow included in the field of radiation.
Materials and Methods
Methods of collection of data
The study is conducted on histological proven cases of cervical cancer attending the department of Radiation oncology at Kerudi Cancer Hospital, Bagalkot, Karnataka, India.
Sample Size
The sample size has been estimated in consultation with a biostatistician based on previous year case load and the sample size is 25.
Inclusion criteria
All patients histologically diagnosed as cervical cancer.
Age: 25-65 years.
KPS ≥70.
Hemoglobin level ≥10gm%.
Platelets count ≥1 lakh.
Total leukocyte count ≥ 4000/cumm.
Exclusion criteria
Previous radiation or chemotherapy. Concomitant malignancy.
Methods
Patients with histologically proven cervical cancer are treated on 6MV linear accelerator with a Radical intent with concurrent chemotherapy using cisplatin (50 mg) weekly. The planning CT was done for all the patients before the treatment and contouring the pelvic bone marrow (ilium, ischium, pubis, sacrum), lumbar spine from L5- ischial tubersoities and proximal femora extending from superior border of femoral heads to the inferior border of ischial tuberosity (Plate 1) apart from other organs at risk like Rectum, Bladder etc were done. Evaluation of plan included the documentation of bone marrow included in the field of irradiation and dose received to it. Hematological toxicity was assessed on weekly basis by recording the HB, Neutrophil, Lymphocyte, ANC and Platelets using RTOG common toxicity criteria version 2 (Table 1).
| Parameters | Grade 1 | Grade 2 | Grade3 | Grade4 |
| TLC (1000/µl): | 3000-<4000 | 2000-<3000 | 1000<2000 | <1000 |
| ANC (1000/µl): | 1500-<1900 | 1000-<1500 | 500-<1000 | 500 |
| HB (gm/dl): | 9.5-11 | 7.5-<9.5 | 5-<7.5 | <5 |
| PLT (1000/µl): | 75000-<100000 | 50000-<75000 | 25000-<50000 | <25000 |
At the occurrence of grade III toxicity the treatment in the form of Blood transfusion, Granulocyte monocyte colony stimulating factors and giving gap in the treatment were considered. The patients were assessed throughout the entire duration of treatment and the last assessment was done two weeks after irradiation.
Statistical Methods
Descriptive statistical analysis has been carried out in the present study. Results on continuous measurements are presented on Mean SD (Min-Max) and results on categorical measurements are presented in Number (%). Significance is assessed at 5% level of significance. Analysis of covariance of toxic levels for adjusting the maximum dose, between two groups of treatment measured, Analysis of variance has been used to find the significance changes in toxic levels at different levels of volume of dose for different time periods.
Significant figures
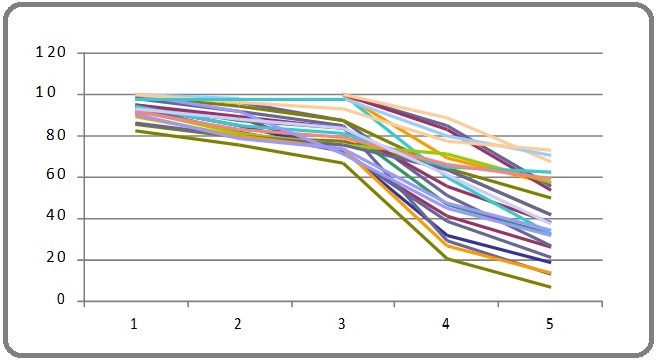
+ Suggestive significance (P value: 0.05 * Moderately significant (P value: 0.01 ** Strongly significant (P value: P≤0.01) The Statistical software namely SAS 9.2, SPSS 15.0, Stata 10.1, MedCalc 9.0.1 and Systat 12.0 were used for the analysis of the data and Microsoft word and Excel have been used to generate graphs (Table 2, 3, 4 and 5). Table 2. Analysis of Estimates of Hemoglobin Levels, Platelet Counts, Total Counts and ANC for Toxicity Levels During the Treatment Period According to Conventional or 3DCRT with and without Adjusting for Maximum Dose. Table 3. Estimates of Toxicity based on Hemoglobin, Platelet counts, Total count, ANC and Lymphocyte. Table 4. Evaluation of Toxicity in Relation to Volume at Different Dose Over the Period of Treatment. Table 5. Evaluation of Volume/Dose According to Toxicity (Grades) in Weeks. A prospective clinical study consisting of 25 patients histopathologically proven cervical cancer is done to assess the hematological toxicity during concurrent chemo radiation in patients with cervical cancer and to correlate the hematological toxicity with the dose and volume of bone marrow included in the field of radiation is attempted. Age group of patients ranged from 38-81 yrs with a mean of 54 yrs. Most of the patients were in the early stage with stage IIB accounting for 48 % (12) of 25 patients. One patient had Stage IB, four had Stage IIIB, six were treated with Post Operative intent and two had Vault recurrence (Table 6). Table 6. Age distribution, Stage wise Distribution, Treatment Received, Chemotherapy Cycles, Corner Shielding in Conventional Technique, Blood Transfusion, Gap in Chemotherapy (1-4 days). All patients received radiation dose ranging from 4500 to 5040 cGy over 25 to 28 fractions with concurrent weekly chemotherapy of cisplatin 50 mg. Fourteen patients underwent conventional technique and 11 with 3DCRT technique. All patients received radiation without any gap. Hematological toxicity in the form of Anaemia ie Grade I was seen in 19 patients, Grade II in 12 patients and Grade III in 2 patients. Eight patients had Grade I at 1st, 2nd and 3rd weeks, 11 patients in 4th week and 13 patients in the 5th week. However at 2 weeks after treatment only 7 patients continued to have Grade I. Grade II anemia was observed from 2nd week onwards and persisted in 3 patients at 2 weeks after treatment. Grade III was observed in 1 patient at 5th week and in another patient at 2 weeks after treatment (Figure 1). Figure 1. Showing the Graphical Representation of the Hemoglobin Levels During the Treatment Period. Seven patients received blood transfusion. Among them one patient had Grade III, one had Grade II anemia during 3rd, 4th and 5th week of treatment. The remaining three received blood transfusion before the commencement of RT. Twelve patients had gap in chemotherapy it ranged from 2-4 days. The reason for the gap were grade III anemia in one and rest of them had gastrointestinal toxicity in the form of vomiting, loose stools etc. Thirteen patients received 5 cycles of chemotherapy, 10 patients received 4 and 2 received only 3 cycles. The reason for only 3 cycles was acute enteritis. None of the patients encountered WBC or Platelets toxicity. Though the mean difference is large at different levels of volume, the significant is not achieved is just because of sample size (sample size distribution at each level of volume is uneven and small), the results give evidence of trend about the how the toxicity changes at different levels of volume for dose range and each time. P value are not very much important, only mean changes is important to measure trend the relationship, to measures changes in toxic levels at different levels of volume, at different dose across the different time periods. A prospective clinical study consisting of 25 patient’s of histopathologically proven cervical cancer is included to assess the hematological toxicity during concurrent chemo radiation in patients with cervical cancer and to Correlate this hematological toxicity with the dose and volume of bone marrow included in the field of radiation. Age group of patients ranged from 38-81 yrs with a mean of 54 yrs. According to various other authors the mean age were ranging from 37-52 yrs [5]. Most of the patients in our study were in the early stage with stage IIB accounting for major of 48% (12) of 25 patients and a similar occurrence of 45% is noted as per different authors [6]. All patients received radiation dose ranging from 4500 to 5040 cGy over 25 to 28 fractions with concurrent weekly chemotherapy of cisplatin 50mg. Similar studies done by authors used RT dose varying from 4300 -5000 cGy over 25-28 fractions but chemotherapy dose of 40mg/m2 of cisplatin was used [7]. Hematological toxicity in the form of anemia was noted in 80% of our patients. GradeI was noted in 19 (95%) patients, Grade II in 12 (60%) patients and Grade III-2 (10%) patients at various points of time during treatment. Among the patients who developed Grade III toxicity, one patient was in the 5th week of CTRT and hence the 5th cycle of cisplatin was withheld while the second patient developed at 2weeks following CTRT. Others authors Bhavaraju et al noted anemia in 62.9% overall, Grade I in 51.1% and Grade II in 11.4% of patients, Grade III, IV were not present [6]. Singh et al noted the similar Grade I, II in 75% and 55% and in study by Shibita et al. [8] Grade III, IV in 50% and 14% higher toxicity is because of 70 mg/m2 of cisplatin dose along with 5-FU. Aich et al noted Grade 0 in none and Grade I, II, III in 54%, 18% and 6% respectively [7]. Rose et al noted Grade I, II, III, IV in 7%, 15%, 5%, 2% patients with concurrent chemo radiation with cisplatin dose of 40mg/m2 [1]. In GOG study by keys et al noted Grade 0, I, II, III, IV in 80%, 0.9%, 0.8%, 0.16% and 0% respectively [9]. In study by Peters ET al in SWOG trial, CT with cisplatin 70mg/m2 with 5FU Grades I, II, III and IV anemia in RT+CT were 23%, 22%, 0.2% and 0% [10]. None of the patients in our study had thrombocytopenia. However Bhavaraju et al did observe Grade 0, I, II and III toxicity in 82.9%, 14.3%, 2.8% and 0 patients respectively [6]. Aich et al grade 0 in 36% and Grade I, II, III in 55%, 9% and 0% respectively [7]. Rose et al noted Grade 0, I, II, III, IV in 44%, 0.8%, 0.2%, 0.1% and 0% of patients respectively [1]. Peters et al noted Grades I, II, III, IV thrombocytopenia in 22%, 0.18, 0.08% and 0% respectively [10]. None of the patients in our study had TLC toxicity. In a study by Bhavaraju et al 28 leucopenia noted with Grade I in 20% and Grade II 31.4% [6]. Aich et al Grade 0 in 44% and Grade I, II, III in 33%, 16% and 7% respectively [7]. Rose et al noted Grade 0, I, II, III, IV in 19%, 0.9%, 14%, 11%, 0.16% patients [1]. Peters et al noted Grades I, II, III, IV leucopenia in 13%, 38%, 32% and 0.02% respectively [10]. None of the patients in our study had ANC toxicity. However in the study by Bhavaraju et al an overall of 51.4% had fall in ANC counts with Grade 0, I, II and III were found to be in 48.6%, 20%, 31.4% and 0% patients respectively [6]. Twelve patients had a gap in chemotherapy the gap ranged from 2-4 days.11 patients had HT and one patient had severe vomiting and burning micturation. In other studies Abu-Rustum 29.2% of patients had incomplete chemotherapy,13% due to Hematological toxicity [11]. In study by Bhavaraju et al interruptions in chemo radiotherapy for a period of 1-4 days was observed in 57% patients for the reason of lack of transportation and he patient being unwell and sick [6]. Aich et al treatment in general was delayed by a week due to HT during CTRT [7]. In our study thirteen (52%) patients received 5 cycles of chemotherapy, 10 (40%) patients received 4 cycles of chemotherapy and 2 (0.08%) patients received 3 cycles. Among the patients who received only 3 cycles, one patient developed jaundice the cause of which was not known and the other lady had severe vomiting and so the remaining chemotherapy was not contemplated. In the study by Abu-Rustum et al 10.8% of patients received six cycles of cisplatin but majority (60%) received planned five courses of cisplatin. Serkies et al noted 55% did not receive the planned five cycles of cisplatin due to treatment related hematological toxicity (31%) and non compliance due to delayed first cycle administration or omission of a cycle for reason other than toxicity [12]. Myrna et al only 67% of patients receive the six planned courses of weekly cisplatin. Keys et al one (0.55%) patient received 2 cycles and all other (99.45%) received 4-6 cycles of chemotherapy. Rose et al 0.6%, 1.1%,1.1%,4%,10.2%,33.5%,49.4% patients received 1,2,3,4,5 and >6 cycles of cisplatin chemotherapy respectively [13]. Fourteen patients underwent conventional technique and 11 with 3DCRT technique and there was no significant difference in the toxicities as far as these techniques were considered and there are no studies comparing the toxicities associated with these techniques however in a study by Loren et al BMS-IMRT were compared to conventional (Four field and AP/PA technique) and a significant bone marrow was spared thereby preventing HT [5]. In our study, anemia was observed also in patients who had corner shielding, however it was not statistically significant. None of the available studies have used corner shielding for preventing the HT. Mitchell et al have used customized shielding for pelvic RT, however the associated toxicities have not been documented corner shielding with custom blocks or MLC were mainly used to reduce enteritis [14]. The various volumes of bone marrow were compared with those of other authors and is as shown in the Table below: (Table 7). Table 7. Showing Various Bone Marrow Volumes According to the Radiation Techniques. Our observations are: 1) V4,V10,V20 Gy is least in AP/PA plan because of only two fields with majority of the bone marrow being outside the field of radiation. 2) All the volume are lesser in the four-field than IMRT because in IMRT multiple fields are used, so in low dose region especially, more of bone marrow volume is receiving greater radiation dose than in four-field box technique. 3) In IMRT-BMS bone marrow can be set as a constraint and then reduction in volume can be accomplished (Table 8). Table 8. Percentage of Bone Marrow Receiving Various Doses. Regarding the toxicities that we observed in our study Grade II Anaemia in 12 and Grade III in 2 patients is probably attributed to cisplatin chemotherapy as it did not correlate with the bone marrow volume in the field of irradiation. Using statistical methods, an effort was made to analyze the hematological toxicity adjusting for that week’s maximum dose the same was analysed without adjusting the maximum dose. This was done because not only the volume was different for different patients but also the dose received on the day of assessing the toxicity was different. However we did not observe any difference between the two groups. We did have certain limitations in our study. The sample size is very small and hence further studies enrolling a large number of patients are required to see if the same results can be duplicated. We have used a flat dose of 50 mg Cisplatin and not the recommended which is 40 mg/m2. Our last assessment of toxicity was at two weeks after completion of treatment and hence it is difficult to comment on the delayed hematological toxicity that is observed with cisplatin. Bone marrow contouring was done entirely from lumbosacral junction (L5) to ischial tuberosity. Contouring different regions like ileum, ischium, and pubis separately will probably help us to understand the toxicity profile better. In conclusion, concurrent chemo radiation for cervical cancer is safe, can be completed as scheduled and is associated with minimal hematological toxicity in the form of anemia and no leucopenia or thrombocytopenia. Chemo radiation induced anemia requiring blood transfusion is uncommon. The volume of bone marrow in the field of irradiation does not correlate with the clinical occurrence of acute hematological toxicity as far as IMRT/3DCRT techniques are considered. Minimal toxicity associated, is probably contributed by concurrent cisplatin administration.
Summary
A total of 25 patients, undergoing concurrent chemo radiation for cervical cancer were assessed weekly once and at two weeks after treatment for hematological toxicity. An effort was made to correlate the volume of bone marrow included in the field of irradiation as well as the dose received by it to the occurrence of anemia, leucopenia and thrombocytopenia. 80% of patients in the study had changes in hemoglobin levels including all the grades. Anaemia of Grade I was seen in 95%,Grade II in 60% and Grade III in 10% of cases at various times during treatment. Grade IV anemia was not observed. No changes in the ANC, Platelets and TLC were noted. The hemoglobin toxicity when compared to the dose and volume in the field of irradiation was not correlating and probably is attributed to cisplatin chemotherapy rather than radiation. Abstract has been accepted as a virtual poster at the upcoming AACR Virtual Special Conference on Radiation Science and Medicine on March 2-3, 2021. 1. IM, RS conceived and designed the experiment, made critical revisions, and approved the final version. RS and IM analyzed the data and wrote the first draft of the manuscript. RS and IM contributed to the writing of the manuscript. RS and IM agree with the manuscript results and conclusions. IM and RS jointly developed the structure and arguments for the paper. All authors reviewed and approved the final manuscript.
Statistical software
Unadjusted for maximum dose
Adjusted for maximum dose
Conventional
3DCRT
P value
Conventional
3DCRT
P value
Hemoglobin (gm/dl)
Before RT
11.64±0.29
11.59±0.44
0.919
11.59±0.33
11.66±0.37
0.9
Week I
11.89±0.33
11.45±0.51
0.449
11.87±0.39
11.47±0.45
0.51
Week II
11.43±0.3
10.59±0.55
0.168
11.36±0.38
10.67±0.43
0.246
Week III
11.14±0.36
10.79±0.48
0.564
11.07±0.38
10.87±0.43
0.738
Week IV
10.55±0.3
10.68±0.3
0.76
10.49±0.27
10.75±0.31
0.533
Week V
10.28±0.34
10.05±0.34
0.639
10.21±0.31
10.13±0.35
0.867
After RT
10.8±0.23
10.61±0.42
0.68
10.73±0.28
10.69±0.32
0.93
Platelet count
Before RT
3.70±0.29
3.44±0.46
0.63
3.67±0.35
3.47±0.40
0.712
Week I
3.72±0.32
3.12±0.27
0.181
3.72±0.29
3.12±0.33
0.199
Week II
2.82±0.24
2.56±0.28
0.497
2.81±0.25
2.58±0.28
0.55
Week III
2.51±0.23
2.43±0.15
0.782
2.51±0.20
2.43±0.23
0.794
Week IV
2.38±0.22
2.18±0.14
0.477
2.37±0.19
2.19±0.21
0.564
Week V
2.29±0.19
2.12±0.14
0.509
2.27±0.16
2.15±0.19
0.634
After RT
3.00±0.32
2.43±0.17
0.155
2.99±0.26
2.45±0.29
0.191
Total counts
Before RT
8728.57±871.48
8918.18±668.49
0.87
8645.34±767.78
9024.12±867.59
0.748
Week I
6139.50±456.31
6726.36±687.72
0.469
6152.97±542.96
6709.22±613.55
0.507
Week II
5655.71±537.19
6330.91±750.43
0.46
5668.51±612.81
6314.63±692.47
0.495
Week III
4485.00±475.26
5693.64±639.66
0.135
4465.17±530.57
5718.87±599.54
0.134
Week IV
4901.43±766.29
4359.09±421.83
0.571
4910.21±644.37
4347.91±728.14
0.571
Week V
4865.71±646.72
4369.09±510.43
0.569
4887.69±585.23
4341.12±661.30
0.545
After RT
4898.57±673.16
5408.18±558.45
0.58
4898.27±619.47
5408.56±700.01
0.593
ANC
Before RT
5748.71±616.58
6152.36±618.23
0.653
8645.34±767.78
9024.12±867.59
0.748
Week I
4378.64±388.04
4700.36±638.33
0.657
6152.97±542.96
6709.22±613.55
0.507
Week II
4199.14±527.04
4689.91±628.64
0.553
5668.51±612.81
6314.63±692.47
0.495
Week III
3251.79±411.21
4261.45±561.92
0.151
4465.17±530.57
5718.87±599.54
0.134
Week IV
3569.64±574.9
3192.55±377.32
0.611
4910.21±644.37
4347.91±728.14
0.571
Week V
3569.57±576.61
3199.73±481.75
0.64
4887.69±585.23
4341.17±661.30
0.545
After RT
3205.14±483.62
3744.18±493.5
0.449
4898.27±619.47
5408.58±700.05
0.593
Hemoglobin
Platelet count
Total count
ANC
LYM
Before RT
11.62±1.22
3.58±1.28
8812±2795.85
5926.32±2162.54
1957.84±721.14
Week I
11.70±1.43
3.45±1.09
6397.72±1958.35
4520.2±1742.41
1273.76±592.81
Week II
11.06±1.49
2.7±0.91
5952.8±2210.53
4415.08±1994.87
883.72±404.18
Week III
10.98±1.44
2.47±0.72
5016.8±1990.76
3696.04±1729.49
754.76±479.48
Week IV
10.61±1.04
2.29±0.69
4662.8±2311.7
3403.72±1787.57
682.80±265.00
Week V
10.18±1.2
2.22±0.61
4647.2±2104.56
3406.84±1902.66
711.76±423.86
After RT
10.72±1.11
2.75±0.98
5122.8±2220.9
3442.32±1721.77
1168.44±690.64
P value
<0.001**
<0.001**
<0.001**
<0.001**
<0.001**
Mean Changes of hemoglobin from baseline
Dose
At Week I
At Week II
At Week III
At week IV
At week V
After RT
V4
<200
-
-
-
-
-
-
201-300
0.7
1.05
1
0.43
1.1
0.1
>300
-0.22
0.47
0.57
1.12
1.51
1.06
P value
0.106
0.368
0.485
0.186
0.516
0.242
V10
<200
-
-
-
-
-
-
201-300
0.56
0.88
0.82
0.84
1.42
0.68
>300
-0.24
0.48
0.59
1.06
1.45
0.96
P value
0.131
0.502
0.687
0.662
0.959
0.713
V20
<200
0.5
1.5
1.2
1.6
2.7
0.4
201-300
0.58
0.73
0.73
0.65
1.1
0.75
>300
-0.24
0.48
0.59
1.06
1.45
0.96
P value
0.327
0.678
0.863
0.628
0.462
0.917
V30
<200
0.13
0.76
0.59
0.54
1.05
-0.1
201-300
-0.42
0.43
0.67
1.19
1.97
1.61
>300
0.2
0.42
0.66
1.6
1.18
1.5
P value
0.419
0.795
0.987
0.092+
0.159
0.014*
V40
<200
0.02
0.63
0.69
0.85
1.32
0.71
201-300
-0.25
0.45
0.87
1.5
2.07
1.55
>300
-0.35
0.3
-0.55
0.9
0.65
0.6
P value
0.816
0.906
0.283
0.372
0.224
0.485
V4
<200
-
-
-
-
-
-
201-300
-0.17
0.79
1.34
1.48
1.71
1.04
>300
0.19
0.9
1.06
1.26
1.3
0.79
P value
0.551
0.853
0.673
0.73
0.491
0.761
V10
<200
-
-
-
-
-
-
201-300
-0.12
0.7
1.14
1.37
1.57
0.85
>300
0.19
0.92
1.1
1.27
1.32
0.83
P value
0.576
0.661
0.951
0.866
0.645
0.978
V20
<200
-1.61
-0.57
0.37
0.94
1.26
-0.35
201-300
0.26
1.01
1.33
1.48
1.64
1.15
>300
0.19
0.92
1.1
1.27
1.32
0.83
P value
0.261
0.351
0.779
0.906
0.859
0.664
V30
<200
0.34
1.17
1.63
1.7
1.81
1.55
201-300
0.05
0.65
0.67
0.82
0.84
0.4
>300
-0.14
0.75
0.93
1.42
1.52
0.26
P value
0.698
0.509
0.178
0.208
0.113
0.121
V40
<200
0.26
0.88
1.15
1.24
1.34
0.91
201-300
-0.1
0.97
0.95
1.15
1.18
0.89
>300
-0.28
0.54
1.2
2.17
2.11
-0.02
P value
0.691
0.879
0.932
0.529
0.57
0.698
V4
<200
-
-
-
-
-
-
201-300
1327
1666.25
1730.5
2664.75
1898
2342.75
>300
1421.19
1481.71
2325.48
2495.52
2637.86
2510.9
P value
0.946
0.907
0.703
0.911
0.672
0.902
V10
<200
-
-
-
-
-
-
201-300
1047
1335.6
1098.4
2358.8
1696.8
1795.2
>300
1495.9
1555.15
2513.25
2563.55
2725.15
2656.2
P value
0.724
0.88
0.318
0.882
0.52
0.488
V4
<200
-
-
-
-
-
-
201-300
0.7
1.05
1
0.43
1.1
0.1
>300
-0.22
0.47
0.57
1.12
1.51
1.06
P value
0.106
0.368
0.485
0.186
0.516
0.242
V10
<200
-
-
-
-
-
-
201-300
0.56
0.88
0.82
0.84
1.42
0.68
>300
-0.24
0.48
0.59
1.06
1.45
0.96
P value
0.131
0.502
0.687
0.662
0.959
0.713
V20
<200
0.5
1.5
1.2
1.6
2.7
0.4
201-300
0.58
0.73
0.73
0.65
1.1
0.75
>300
-0.24
0.48
0.59
1.06
1.45
0.96
P value
0.327
0.678
0.863
0.628
0.462
0.917
V30
<200
0.13
0.76
0.59
0.54
1.05
-0.1
201-300
-0.42
0.43
0.67
1.19
1.97
1.61
>300
0.2
0.42
0.66
1.6
1.18
1.5
P value
0.419
0.795
0.987
0.092+
0.159
0.014*
V40
<200
0.02
0.63
0.69
0.85
1.32
0.71
201-300
-0.25
0.45
0.87
1.5
2.07
1.55
>300
-0.35
0.3
-0.55
0.9
0.65
0.6
P value
0.816
0.906
0.283
0.372
0.224
0.485
V4
<200
-
-
-
-
-
-
201-300
-0.17
0.79
1.34
1.48
1.71
1.04
>300
0.19
0.9
1.06
1.26
1.3
0.79
P value
0.551
0.853
0.673
0.73
0.491
0.761
V10
<200
-
-
-
-
-
-
201-300
-0.12
0.7
1.14
1.37
1.57
0.85
>300
0.19
0.92
1.1
1.27
1.32
0.83
P value
0.576
0.661
0.951
0.866
0.645
0.978
Toxicity level
V4
V10
V20
V30
V40
Week1
· GrI
94.71±6.21
89.56±7.64
80.81±10.70
46.14±15.46
29.35±15.89
Week2
· GrI
96.36±4.04
92.13±6.12
84.84±10.92
56.27±9.95
38.69±12.74
· GrII
90.08±10.99
83.62±11.59
75.89±12.92
24.94±6.22
9.97±4.35
Week3
· GrI
93.22±6.49
86.26±8.18
78.82±10.51
43.02±14.90
26.51±12.19
· GrII
93.87±4.61
89.46±8.24
82.42±12.86
48.26±18.39
33.74±18.58
Week4
· GrI
92.22±4.62
84.84±6.57
77.30±8.45
48.54±18.41
36.20±20.64
· GrII
100.00±0.00
100.00±0.00
100.00±0.00
72.71±14.80
52.28±17.96
Week5
· GrI
93.16±5.47
86.39±7.15
78.79±8.59
48.21±17.14
32.44±18.06
· GrII
97.28±3.14
93.06±7.15
89.35±8.59
51.95±16.34
35.19±16.02
· GrIII
94.09
85
81.38
65
62.5
After RT
· GrI
95.26±5.27
89.61±7.96
83.49±11.99
56.59±16.74
38.61±14.02
· GrII
97.96±3.46
92.62±9.71
86.56±13.91
53.11±15.50
35.09±19.26
· GrIII
91.1
88.79
72.06
41.09
25.97
Results
Age in years
Number of patients
%
31-40
3
12
41-50
8
32
51-60
7
28
>60
7
28
Total
25
100
( Mean ± SD: 53.72±11.56)
Diagnosis
(n=25)
Cancer cervix
· Stage IB
1
4
· Stage IIB
12
48
· Stage IIIB
4
20
CA. Cervix Post Op
6
16
VAULT recurrence
2
8
Treatment
(n=25)
Conventional
14
56
3D CRT
11
44
Chemotherapy cycles
(n=25)
3
2
8
4
10
40
5
13
52
Corner Shielding in conventional technique
(n=14)
No
11
78.57
Yes
3
21.42
Blood transfusion
(n=25)
No
19
76
Yes
6
24
Gap in chemotherapy day
(n=25)
No
13
56
Yes
12
48
Discussion
Dose received by bone marrow volume
Four-field box (Our study) (%)
AP/PA plan (Loren) [5] (%)
Four-field box (Loren) [5] (%)
IMRT (Roeske) [15] (%)
IMRT- BMS (Loren 68) (%)
V4
91.2
72.4
99.6
100
90.4
V10
89.3
66.9
97.3
100
76.5
V20
83.4
62.9
92.7
96
57.5
V30
54.8
59.1
59.9
76
46.1
V40
40.1
54.1
48.9
49
33.7
Percentage of bone marrow receiving RT dose
Range in percentage
Mean in percentage
4 Gy
82.31-100
91.2
10 Gy
78.54-100
89.3
20 Gy
66.76-100
83.4
30 Gy
20.54-88.87
54.8
40 Gy
6.89-73.35
40.1
Acknowledgements
Conflicts of Interest
Author Contributions
References
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2021
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times