Real World Data of Response of Trastuzumab Based Chemotherapy in Locally Advanced HER2 Positive Breast Cancer from a Developing Country
Download
Abstract
Background: Data regarding pathologic response of Trastuzumab based chemotherapy in locally advanced HER2 positive breast cancer in neoadjuvant setting is scarce.
Methods: A retrospective analysis was conducted from January 2014 to January 2019 at a tertiary cancer care centre in North India and 81 breast cancer patients who underwent neoadjuvant chemotherapy were included. The clinical and pathologic characteristics, response, toxicity and survival data was collected, collated and analyzed.
Results: The most commonly observed tumor characteristics at baseline were clinical stage T4 (72.8%), nodal stage N2 (40.7%), invasive ductal carcinoma on histology (98.8%), grade 3 (66.7%) and hormone receptor negativity (54.3%). In terms of post treatment characteristics, a higher incidence of partial response (55.6%), post treatment tumor stage ypT0 (45.7%), nodal status ypN0 (54.3%), absence of extracapsular invasion (77.8%) and absence of pathologic complete response (pCR, 63%) were observed. pCR was attained in 30 patients and was most commonly associated with clinical tumor stage T4 (26/30), nodal stage N2-N3 (19/30), grade 3 (21/30) and hormone receptor negativity (20/30). Altogether, 19.75% had grade 3/4 adverse events. At 6 years, 86% v/s 61% patients were disease free (p=0.037) and 93% v/s 79% patients (p=0.181) were alive in the pCR and no pCR groups, respectively.
Conclusion: Even in locally advanced breast cancer (LABC), Trastuzumab had good response in terms of pCR and survival outcomes. Thus, one can be encouraged to use this single HER2 blockade if dual blockade is not feasible in HER2 positive LABC in the neoadjuvant setting.
Introduction
Breast cancer is the most common cancer in women, both in the developed and the developing countries [1]. Though breast cancer was thought to be a disease of the developed countries, its incidence has been gradually increasing in the developing countries (likely due to westernization and increasing life expectancy) [1]. Breast cancer is now the most common cancer in the Indian women, both rural and urban. In 2018, about 162,468 women were newly diagnosed with breast cancer and 87,090 women succumbed in India [2], indicating 50% mortality. One of the main reasons for the high mortality is procuring medical help in advanced stages of the disease. They mainly present as locally advanced breast cancer (LABC) which accounts for up to 50% of all newly diagnosed cases of breast cancer [3], while these numbers are 5-15% in the developed countries [4]. Management of LABC is a clinical challenge in terms of offering appropriate oncological outcome and maintaining a good quality of life.
The presence or absence of residual invasive cancer [known as a pathological complete response (pCR)] after neoadjuvant chemotherapy (NACT) is a strong prognostic risk factor and surrogate endpoint for recurrence free survival, especially in Human Epidermal growth Receptor 2 (HER2) positive breast cancer [5]. The current preferred therapy for HER2 positive breast cancer in the neoadjuvant setting is chemotherapy with anti-HER2 targeted therapy, i.e. dual blockade with trastuzumab with pertuzumab/lapatinib or single blockade with trastuzumab alone. These recommendations are based on the higher rates of pCR with addition of HER2 targeted agents with chemotherapy and with evolving data, the preferred regimen now is dual anti HER2 therapy, which has further shown to increase pCR [6-11]. However, due to financial constraints, single-agent Trastuzumab is preferred as anti HER2 targeted therapy in neoadjuvant setting for breast cancer in a developing country like India. The present study was therefore conducted to evaluate the response of Trastuzumab based chemotherapy in locally advanced HER2 positive breast cancer in a single centre in a developing country.
Materials and Methods
A retrospective observational study was conducted during the period January 2014 to June 2019 in the Department of Medical Oncology at a tertiary cancer care centre in North India. A total of 130 female patients in the age group of 18-65 years with Eastern Cooperative Oncology Group (ECOG) performance status of 0-1 having stage III HER2 positive LABC were screened for the study, of which, 81 patients who underwent neoadjuvant chemotherapy (NACT) were included in the detailed analysis. The consort diagram is presented in Figure 1.
Figure 1. Consort Diagram.
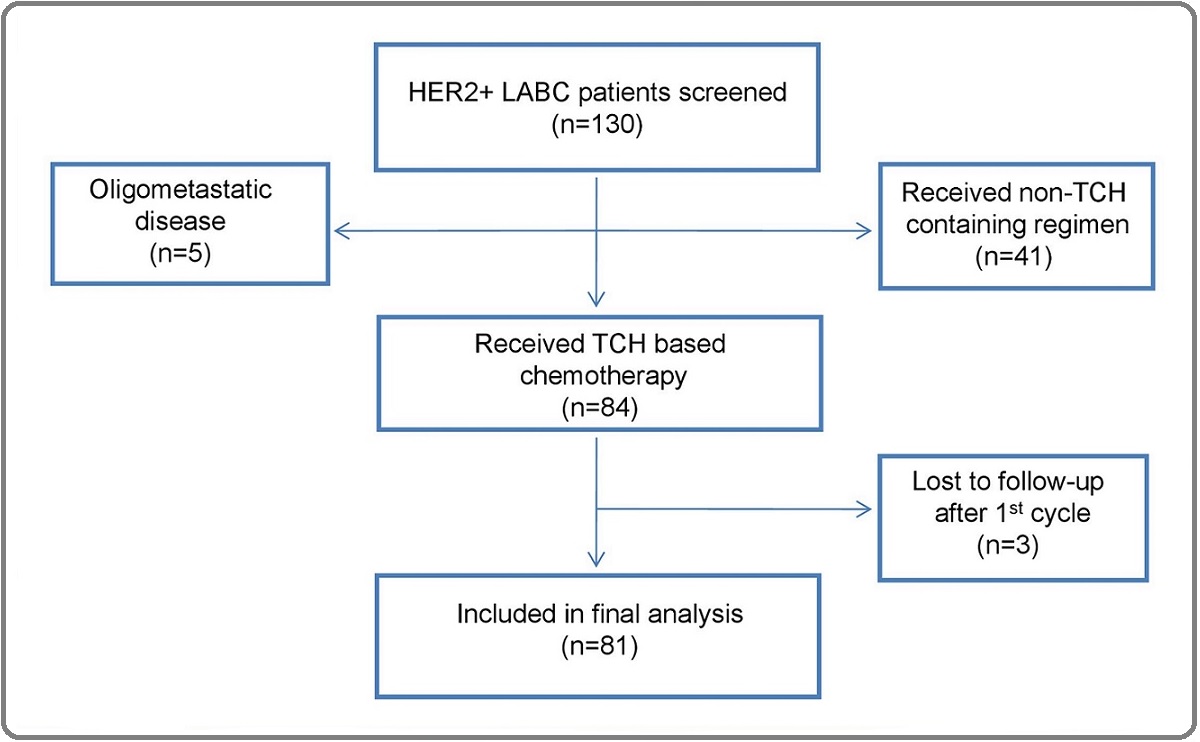
Only histologically confirmed cases of primary invasive carcinoma of the breast were included in the study. HER2-positivity was defined as IHC 3+ or FISH positivity in cases of IHC 2+. The present study was approved by the Institutional Review Board of Rajiv Gandhi Cancer Institute & Research Centre, Delhi, India (vide letter no. RGCIRC / IRB / 74 / 2017) and was given a waiver from the informed consenting process. The study was conducted as per the Helsinki Declaration.
The clinical and pathologic characteristics of all the patients were recorded. Related to the treatment details for LABC, the neo-adjuvant chemotherapy (NACT) consisted of 6 cycles of Trastuzumab based chemotherapy followed by response evaluation. Surgery was done after 6 cycles of chemotherapy depending on the clinical & radiological response. Trastuzumab was continued for total duration of one year (continued three weekly trastuzumab through post-operative and radiation therapy time). Doses and drugs used in trastuzumab based chemotherapy were (with growth factor support): Inj. Trastuzumab 8 mg/kg 1st dose, followed by 6mg/kg, Inj. Docetaxel: 75 mg/m2, Inj. Carboplatin as per AUC 5 (q 3 weekly) as per the BCIRG-006 (5). Response was assessed clinically during each visit and radiological response assessed using response evaluation criteria for solid tumors (RECIST v1.0) at the end of 3 and 6 cycles, prior to surgery. Prior to starting chemotherapy titanium clips were placed within the tumor for future resectability. The pathologic response to chemotherapy was determined by analyzing the surgical tumor specimens. Most patients underwent axillary nodal dissection as they were node positive clinically. The residual tumor size and lymph node status were evaluated to assess the pathologic response to NACT. pCR was defined as no pathologic evidence of a residual invasive carcinoma in the breast or axillary lymph nodes (ypT0-isN0 status). Residual ductal carcinoma in situ (Tis) was included under pCR. Toxicity was assessed at every visit using the National Cancer Institute Common Toxicity Criteria version 4.0 [6].
Statistical analysis
SPSS version 22 for Windows (SPSS Inc, Chicago IL, USA) was used for statistical analysis. The statistical comparisons for quantitative variables was done using unpaired t-test or Mann-Whitney ‘U’ test and for categorical variables, Chi-square or Fisher’s exact test were used as per the nature of data. Survival analysis was performed using the Kaplan Meier method. Log Rank test was used to compare the difference in survival among the groups. A two sided p-value <0.05 was considered as significant.
Results
A total of 81 patients receiving the TCH based chemotherapy were included in the study. Of these, 2 patients could not undergo surgery. The baseline and post treatment characteristics of the patients have been shown in Table 1.
| Characteristics | N |
| Age group | |
| <50 years/ >50 years | 35/ 46 |
| Menopausal status | |
| Premenopausal/ Postmenopausal | 40/ 41 |
| Clinical tumor stage | |
| cT1-T3/ cT4 | 22/ 59 |
| Clinical nodal stage | |
| cN0/ cN1/ cN2/ cN3 | 1/ 31/ 33/ 16 |
| TNM staging | |
| II/ III | 4/77 |
| Histology | |
| Invasive ductal carcinoma/ Others | 80/ 1 |
| Grade | |
| 1/2/3 | 3/24/54 |
| Estrogen receptor | |
| No/ Yes | 45/ 36 |
| Progesterone receptor | |
| No/ Yes | 60/ 21 |
| Hormone receptor | |
| Negative/ Positive | 44/ 37 |
| Clinical response | |
| Complete response/ Partial response/ Progressive disease | 35/ 45/ 1 |
| Post treatment tumor (ypT) | |
| ypT0/ ypT1/ ypT2/ ypT3/ ypT4 | 37/ 18/ 18/ 4/ 2/ 2 |
| Post treatment nodes (ypN) | |
| ypN0/ ypN1/ ypN2/ ypN3/ No surgery | 44/ 23/ 9/ 3/ 2 |
| Extracapsular invasion | |
| No/ Yes/ No surgery | 63/ 16/ 2 |
| Pathologic complete response | |
| No/ Yes | 51/ 30 |
The median age of the patients was 52 years (range 26-75 years). Majority of the patients (60/81) were in the age group of 21-40 years. The most commonly observed baseline characteristics among the patients were age >50 years (56.8%), postmenopausal status (50.6%), clinical tumor stage T4 (72.8%), clinical nodal stage N2 (40.7%), invasive ductal carcinoma on histology (98.8%), grade 3 (66.7%) and hormone receptor negativity (54.3%). With respect to the post treatment characteristics, a higher incidence of partial response (55.6%), post treatment tumor stage ypT0 (45.7%), post treatment nodal status ypN0 (54.3%), absence of extracapsular invasion (77.8%) and absence of pathologic complete response (pCR, 63%) were observed.
Further, a correlation of the baseline characteristics with pathologic complete response was performed (Table 2).
| Characteristics | N | Pathologic complete response | p-value | |
| No | Yes | |||
| n=51 | n=30 | |||
| Age group | 0.486 | |||
| <50 years | 35 | 24 | 11 | |
| >50 years | 46 | 27 | 19 | |
| Menopausal status | 0.646 | |||
| Premenopausal | 39 | 26 | 13 | |
| Postmenopausal | 42 | 25 | 17 | |
| Clinical tumor stage | 0.603 | |||
| cT1-T3 | 22 | 11 | 11 | |
| cT4 | 59 | 33 | 26 | |
| Clinical nodal stage | 0.815 | |||
| cN0-N1 | 32 | 21 | 11 | |
| cN2-N3 | 49 | 30 | 19 | |
| Grade | 0.808 | |||
| 1-2 | 27 | 18 | 9 | |
| 3 | 54 | 33 | 21 | |
| Estrogen receptor | 0.064 | |||
| No | 45 | 24 | 21 | |
| Yes | 36 | 27 | 9 | |
| Progesterone receptor | 0.192 | |||
| No | 60 | 35 | 25 | |
| Yes | 21 | 16 | 5 | |
| Her2 neu | 0.738 | |||
| 2+ | 11 | 8 | 3 | |
| 3+ | 70 | 43 | 27 | |
| Hormone receptor | ||||
| Negative | 44 | 24 | 20 | 0.109 |
| Positive | 37 | 27 | 10 |
A pCR was attained in a total of 30 patients. Also, pCR in the patients was most commonly observed in the age >50 years (19/30), postmenopausal status (17/30), clinical tumor stage T4 (26/30), clinical nodal stage N2-N3 (19/30), grade 3 (21/30) and hormone receptor negativity (20/30). However, the results were not statistically significant.
In terms of adverse events, the chemotherapy regimen TCH was well tolerated in the study population. Growth factor support was used in all the patients. The major adverse events have been shown in Figure 2.
Figure 2. Adverse Events.
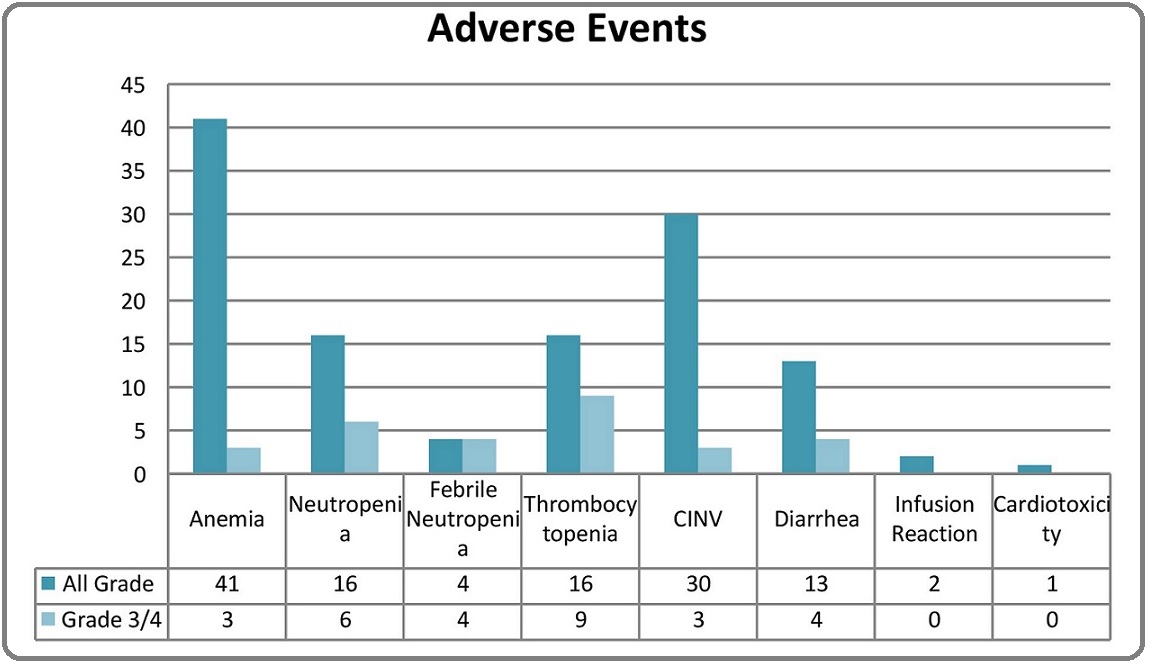
Altogether, there were 16 patients (19.75%) with grade 3/4 adverse events. The most common adverse events were hematological. The most common grade 3/4 event requiring inpatient transfusion and supportive care was thrombocytopenia in 9 patients (11%), but with no episodes of major bleeding. Despite the growth factor support, there were 4 patients with febrile neutropenia, with one patient requiring intensive care in view of septic shock. Uncomplicated grade 3/4 neutropenia was seen in 2 patients. Anemia was seen in 41 (50.61%) patients, with 3 patients with grade 3 or 4 event requiring blood transfusion support. Amongst the non-haematological adverse events, gastrointestinal (GI) adverse events were the most common. Three patients had grade 3 or 4 event and required inpatient supportive care. Hypersensitivity was seen during infusion of Trastuzumab in 2.5% patients which was managed with anti-histamines and steroids without dose modification or discontinuation. There were no grade 3 or 4 infusional reactions. Cardiotoxicity was one of the most fatal adverse event but was seen in 1 patient with underlying coronary artery disease, who had asymptomatic, reversible fall in left ventricular ejection fraction, which recovered without any therapy. There were no grade 3 or 4 events of cardiotoxicity seen in the present study. Among all the patients, 8 (9.9%) patients had delay in chemotherapy dosing with median delay of 3 days due to adverse events. A total of 24 (29.6%) patients had nail changes (discolouration) during therapy which reversed after the completion of therapy. Alopecia was seen in all the patients which reversed after the completion of chemotherapy.
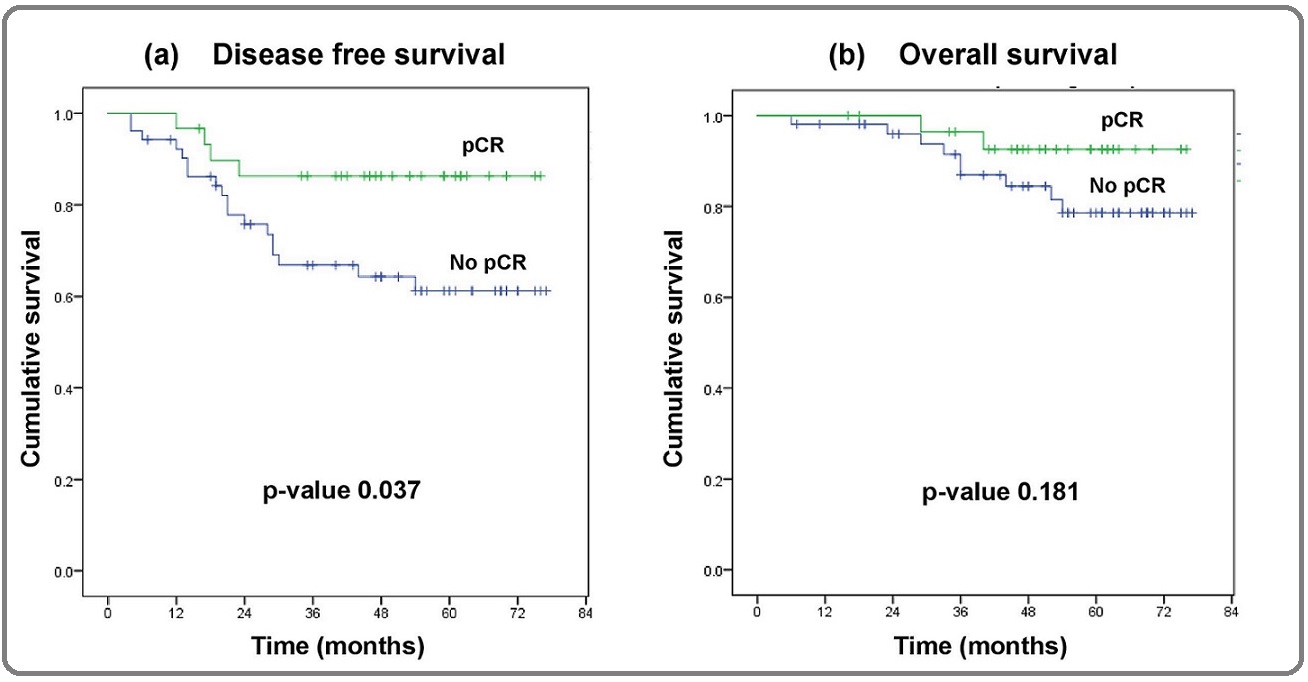
Survival was analyzed for all the patients. At 6 years, 86% v/s 61% patients were disease free (p=0.037) and 93% v/s 79% patients (p=0.181) were alive in the pCR and no pCR groups, respectively (Figure 3).
Figure 3. Survival Analysis.
Discussion
LABC is a major problem in our country with ignorance and late consultations with healthcare. The knowledge about the hormone receptors has brought to light the importance of the key signaling pathways as the dominant drivers of cell proliferation and survival in the majority of breast cancers. HER2 positive breast cancers are aggressive tumors and early tumor control with neoadjuvant chemotherapy helps abate the poor prognosis associated with HER2 positivity [12-13]. There is a paucity of data in HER2 positive breast cancer pertaining to neoadjuvant chemotherapy regimens. The data on Trastuzumab based neoadjuvant regimes are limited and more so in the Asian population.
In the present study, the efficacy and toxicity profile of non-anthracycline Trastuzumab based chemotherapy in the neo-adjuvant setting was recorded in stage II/III (LABC/EBC) HER2+ breast cancer. Out of 81 patients analyzed, 59 (72.8%) patients had tumors either infiltrating the skin or chest wall or both (T4). Nodal positivity was clinically present in more than 90% patients and of these, 60% had N2-/N3 nodal status. In this study, there were more advanced stage tumors as compared to the previous similar studies by Buzdar et al and NOAH trial [14] where there were majority of patients with early breast cancer which could partially explain the comparatively slightly lower pCR rate in this study.
There has been a debate about whether anthracylines be used concomitantly with trastuzumab or not. Various trials in adjuvant setting have shown that non-anthracyline based chemotherapy regimen is doing as good [15], if not better in the adjuvant setting. Recently there has been data on non-anthracycline based chemotherapy in neoadjuvant setting as well but with dual HER2 blockade [5,7,8].
In terms of response, the clinical response rate in our study was comparatively lower to clinical complete response rates of 87% observed by Buzdar et al [16-17]. This difference again could be due to higher proportion of T4 tumors in our study as compared to these studies, which had fewer patients with T4 disease or N2-N3 nodal status. In patients with clinical complete response, 30/35 (87.5%) patients had pCR thereby demonstrating a good concordance between response assessment by MRI/PET-CT after neo-adjuvant chemotherapy and pCR in surgical specimen. Similar concordance has been seen in NOAH trial where out of 87% clinical responses, 81% patients achieved pCR [14].
pCR has been shown to correlate with long term outcome measures such as DFS and OS and hence taken as surrogate marker to evaluate the long term outcome of any chemotherapy regimen. pCR rate in our study was 37.0% and it is comparable to most previous trials using neoadjuvant chemotherapy with Trastuzumab, although the chemo regimens used in those studies are varied. In the largest NOAH trial [14], the pCR rates were 38% similar to our study although in that trial almost doubling of pCR was reported in comparison to non-Trastuzumab containing chemo-regimen. In the Gepar Quattro trial, (EC followed by docetaxel with or without capecitabine in combination with or without trastuzumab), pCR rates were 40% which were not significantly different with different chemotherapeutic regimes [18]. Some smaller studies have reported lower pCR rate of 26% like by Pierga et al [19], while some reported very high pCR rate upto 67% like in study by Buzdar et al [16-17]. In our study pCR rate was comparable to above mentioned studies despite of fact that three quarter patients in this study being advanced T4 tumors and we used non-anthracyclines based chemo regimen. A longer follow up period is necessary to demonstrate this pCR turning into DFS or OS advantage. An important factor that predicts pCR is the number of anti-HER2 agent that is used [20-22] (Table 3).
| Study | Chemotherapy regimen | pCR rates |
| GeparQuattro [18] | EC → T±X±H | 31.70 |
| TECHNO Trial [32] | EC → TH | 39 |
| NOAH [14] | Varied | 38 |
| Tiwari et al [33] | TCH | 36.30 |
| NeoSphere [21] | TH+ P | 45.80 |
| TRYPAHENA [22] | TCH+P | 66.10 |
| Bayraktar et al [34] | TCH | 36.30 |
| NeoALTTO [35] | Paclitaxel+H+L | 51.30 |
| Present study | TCH | 37 |
From not using any anti Her2 agent to using dual blockade, the pathological complete response increases. In this study, various biological, clinico-pathologic and treatment related factors were evaluated which were expected to predict pCR. Higher grade (III) predicted clinical complete response as well as pCR similar to previously published studies [23-26]. Hormone positivity was seen in 45% patients in this study and ER and PR negative tumors are also associated with higher pCR rates (not statistically significant) as observed in our study and previously published studies [23-29].
The regimen was well tolerated with few adverse events recorded in the present study which were mainly hematological and gastrointestinal. There were 16 patients (19.8%) with grade 3/4 adverse events. Most of these adverse events resolved with few admissions for supportive care. There were 8 patients with dose delay and/or modification due to these adverse events. The adverse events in present study were higher as compared to previous studies [14,30]. Kolberg et al reported no grade 3/4 adverse events with no dose delays or modifications [30]. Comparatively higher adverse events in this study may be attributed to poor baseline nutritional status of Indian patients. Based upon concerns regarding cardiotoxicity, anthracycline-free chemotherapy backbones for trastuzumab have been explored in the adjuvant and in the neoadjuvant setting. The data on efficacy of the non-anthracyclines Trastuzumab containing regimen is limited. Kolberg et al., presented a study of operable HER2 positive breast cancers with TCH regimen in NACT and observed a pCR of 64% in a cohort treated with TCH, the regimen used in the BCIRG-006 trial in the adjuvant setting [31]. Variable pCR rates have been observed in the different studies [32-35].
This study has one of the longest follow up with TCH based chemotherapy in neoadjuvant setting in locally advanced HER2 positive breast cancer. pCR has been shown to correlate with long term outcome measures such as DFS and OS and hence taken as surrogate marker to evaluate long term outcome of any chemotherapy regimen. Thus, regardless of the fact that anthracycline was not used in the chemotherapy backbone, the regimen was well tolerated with good oncological outcome in terms of the response and survival outcomes.
Even in LABC, TCH had good response in terms of pCR and survival outcomes comparable with adjuvant trials. Thus, one can be encouraged to use TCH based chemotherapy in Her2 positive breast cancers in neoadjuvant setting. With the advent of dual HER2 blockade, single HER2 blockade with trastuzumab is still an important choice in developing nations, where dual blockade can be procured by only a handful of patients. Also, patient stratification for appropriate neoadjuvant regimen is important in the light of newer drugs like Pertuzumab and TDM1.
In conclusion, even in LABC, TCH had good response in terms of pCR and survival outcomes comparable with adjuvant trials. Thus, one can be encouraged to use TCH based chemotherapy in Her2 positive breast cancers in neoadjuvant setting. With the advent of dual HER2 blockade, single HER2 blockade with trastuzumab is still an important choice in developing nations, where dual blockade can be procured by only a handful of patients. Also, patient stratification for appropriate neoadjuvant regimen is important in the light of newer drugs like Pertuzumab and TDM1.
Ethics
The present study was approved by the Institutional Review Board of Rajiv Gandhi Cancer Institute & Research Centre, Delhi, India (vide letter no. RGCIRC / IRB / 74 / 2017) and was given a waiver from the informed consenting process. The study was conducted as per the Helsinki Declaration.
Acknowledgements
Funding
No funding
Conflict of interest
The authors have no conflicts of interest to declare.
Author contributions
Conception or design of the work: DCD
Acquisition, analysis, or interpretation of data for the work: SB, PG, CA, PJ, RT, AS, SP, KDC
Drafting the work or revising it critically for important intellectual content: DCD, SB, PG, CA, PJ, RT, AS, SP, KDC
Final approval of the version to be published: DCD, SB, PG, CA, PJ, RT, AS, SP, KDC
References
- Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries Bray Freddie, Ferlay Jacques, Soerjomataram Isabelle, Siegel Rebecca L., Torre Lindsey A., Jemal Ahmedin. CA: A Cancer Journal for Clinicians.2018;68(6). CrossRef
- Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods Ferlay J., Colombet M., Soerjomataram I., Mathers C., Parkin D.M., Piñeros M., Znaor A., Bray F.. International Journal of Cancer.2018;144(8). CrossRef
- Spectrum of Breast Cancer in Asian Women Agarwal Gaurav, Pradeep P. V., Aggarwal Vivek, Yip Cheng-Har, Cheung Polly S. Y.. World Journal of Surgery.2007;31(5). CrossRef
- LOCALLY ADVANCED BREAST CANCER Esteva Francisco J., Hortobagyi Gabriel N.. Hematology/Oncology Clinics of North America.1999;13(2). CrossRef
- Pathological Complete Response and Accelerated Drug Approval in Early Breast Cancer Prowell Tatiana M., Pazdur Richard. New England Journal of Medicine.2012;366(26). CrossRef
- A reduction in the requirements for mastectomy in a randomized trial of neoadjuvant chemoendocrine therapy in primary breast cancer Makris A., Powles T.J., Ashley S.E., Chang J., Hickish T., Tidy V.A., Nash A.G., Ford H.T.. Annals of Oncology.1998;9(11). CrossRef
- Neoadjuvant chemotherapy for operable breast carcinoma larger than 3 cm: A unicentre randomized trial with a 124-month median follow-up Mauriac L., MacGrogan G., Avril A., Durand M., Floquet A., Debled M., Dilhuydy J.M., Bonichon F.. Annals of Oncology.1999;10(1). CrossRef
- A mathematic model for relating the drug sensitivity of tumors to their spontaneous mutation rate Goldie JH, Coldman AJ. Cancer Treat Rep.1979;63(11-12):1727-1733.
- Effect of surgical removal on the growth and kinetics of residual tumor Gunduz N, Fisher B, Saffer EA. Cancer Res.1979;39(10):3861-3865.
- Pathobiology of preoperative chemotherapy Fisher Edwin R., Wang Jiping, Bryant John, Fisher Bernard, Mamounas Eletherios, Wolmark Norman. Cancer.2002;95(4). CrossRef
- Influence of the interval between primary tumor removal and chemotherapy on kinetics and growth of metastases Fisher B, Gunduz N, Saffer EA. Cancer Res.1983;43(4):1488-1492.
- Efficacy of TCH/TEC neoadjuvant chemotherapy for the treatment of HER-2-overexpressing breast cancer CHEN WEICAI, HE JINSONG, SONG SHUFEN, WANG MIN, WU HUISHENG, WANG XIANMING. Oncology Letters.2015;9(4). CrossRef
- Long-term follow-up of HER2-overexpressing stage II or III breast cancer treated by anthracycline-free neoadjuvant chemotherapy Guiu S., Liegard M., Favier L., van Praagh I., Largillier R., Weber B., Coeffic D., Moreau L., Priou F., Campone M., Gligorov J., Vanlemmens L., Trillet-Lenoir V., Arnould L., Coudert B.. Annals of Oncology.2011;22(2). CrossRef
- Neoadjuvant chemotherapy with trastuzumab followed by adjuvant trastuzumab versus neoadjuvant chemotherapy alone, in patients with HER2-positive locally advanced breast cancer (the NOAH trial): a randomised controlled superiority trial with a parallel HER2-negative cohort Gianni Luca, Eiermann Wolfgang, Semiglazov Vladimir, Manikhas Alexey, Lluch Ana, Tjulandin Sergey, Zambetti Milvia, Vazquez Federico, Byakhow Mikhail, Lichinitser Mikhail, Climent Miguel Angel, Ciruelos Eva, Ojeda Belén, Mansutti Mauro, Bozhok Alla, Baronio Roberta, Feyereislova Andrea, Barton Claire, Valagussa Pinuccia, Baselga Jose. The Lancet.2010;375(9712). CrossRef
- Polychemotherapy for early breast cancer: an overview of the randomised trials Early Breast Cancer Trialists’ Collaborative Group. Lancet.1988;352(9132):930-942.
- Significantly Higher Pathologic Complete Remission Rate After Neoadjuvant Therapy With Trastuzumab, Paclitaxel, and Epirubicin Chemotherapy: Results of a Randomized Trial in Human Epidermal Growth Factor Receptor 2–Positive Operable Breast Cancer Buzdar Aman U., Ibrahim Nuhad K., Francis Deborah, Booser Daniel J., Thomas Eva S., Theriault Richard L., Pusztai Lajos, Green Marjorie C., Arun Banu K., Giordano Sharon H., Cristofanilli Massimo, Frye Debra K., Smith Terry L., Hunt Kelly K., Singletary Sonja E., Sahin Aysegul A., Ewer Michael S., Buchholz Thomas A., Berry Donald, Hortobagyi Gabriel N.. Journal of Clinical Oncology.2005;23(16). CrossRef
- Neoadjuvant Therapy with Paclitaxel followed by 5-Fluorouracil, Epirubicin, and Cyclophosphamide Chemotherapy and Concurrent Trastuzumab in Human Epidermal Growth Factor Receptor 2–Positive Operable Breast Cancer: An Update of the Initial Randomized Study Population and Data of Additional Patients Treated with the Same Regimen Buzdar Aman U., Valero Vicente, Ibrahim Nuhad K., Francis Deborah, Broglio Kristine R., Theriault Richard L., Pusztai Lajos, Green Marjorie C., Singletary Sonja E., Hunt Kelly K., Sahin Aysegul A., Esteva Francisco, Symmans William F., Ewer Michael S., Buchholz Thomas A., Hortobagyi Gabriel N.. Clinical Cancer Research.2007;13(1). CrossRef
- Neoadjuvant Treatment With Trastuzumab in HER2-Positive Breast Cancer: Results From the GeparQuattro Study Untch Michael, Rezai Mahdi, Loibl Sibylle, Fasching Peter A., Huober Jens, Tesch Hans, Bauerfeind Ingo, Hilfrich Jörn, Eidtmann Holger, Gerber Bernd, Hanusch Claus, Kühn Thorsten, du Bois Andreas, Blohmer Jens-Uwe, Thomssen Christoph, Dan Costa Serban, Jackisch Christian, Kaufmann Manfred, Mehta Keyur, von Minckwitz Gunter. Journal of Clinical Oncology.2010;28(12). CrossRef
- A multicenter randomized phase II study of sequential epirubicin/cyclophosphamide followed by docetaxel with or without celecoxib or trastuzumab according to HER2 status, as primary chemotherapy for localized invasive breast cancer patients Pierga Jean-Yves, Delaloge Suzette, Espié Marc, Brain Etienne, Sigal-Zafrani Brigitte, Mathieu Marie-Christine, Bertheau Philippe, Guinebretière Jean Marc, Spielmann Marc, Savignoni Alexia, Marty Michel. Breast Cancer Research and Treatment.2010;122(2). CrossRef
- Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): survival outcomes of a randomised, open-label, multicentre, phase 3 trial and their association with pathological complete response de Azambuja Evandro, Holmes Andrew P, Piccart-Gebhart Martine, Holmes Eileen, Di Cosimo Serena, Swaby Ramona F, Untch Michael, Jackisch Christian, Lang Istvan, Smith Ian, Boyle Frances, Xu Binghe, Barrios Carlos H, Perez Edith A, Azim Hatem A, Kim Sung-Bae, Kuemmel Sherko, Huang Chiun-Sheng, Vuylsteke Peter, Hsieh Ruey-Kuen, Gorbunova Vera, Eniu Alexandru, Dreosti Lydia, Tavartkiladze Natalia, Gelber Richard D, Eidtmann Holger, Baselga José. The Lancet Oncology.2014;15(10). CrossRef
- Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomisedmulticentre, open-label, phase 2 trial Gianni L, Pienkowski T, Im Y-H, et al . Lancet Oncol.2012;13(1):25-32.
- Pertuzumab plus trastuzumab in combination with standard neoadjuvant anthracycline-containing and anthracycline-free chemotherapy regimens in patients with HER2-positive early breast cancer: a randomized phase II cardiac safety study (TRYPHAENA) Schneeweiss A., Chia S., Hickish T., Harvey V., Eniu A., Hegg R., Tausch C., Seo J.H., Tsai Y.-F., Ratnayake J., McNally V., Ross G., Cortés J.. Annals of Oncology.2013;24(9). CrossRef
- Impact of treatment characteristics on response of different breast cancer phenotypes: pooled analysis of the German neo-adjuvant chemotherapy trials von Minckwitz Gunter, Untch Michael, Nüesch Eveline, Loibl Sibylle, Kaufmann Manfred, Kümmel Sherko, Fasching Peter A., Eiermann Wolfgang, Blohmer Jens-Uwe, Costa Serban Dan, Mehta Keyur, Hilfrich Jörn, Jackisch Christian, Gerber Bernd, du Bois Andreas, Huober Jens, Hanusch Claus, Konecny Gottfried, Fett Werner, Stickeler Elmar, Harbeck Nadia, Müller Volkmar, Jüni Peter. Breast Cancer Research and Treatment.2010;125(1). CrossRef
- Recommendations from an international expert panel on the use of neoadjuvant (primary) systemic treatment of operable breast cancer: new perspectives 2006 Kaufmann M., von Minckwitz G., Bear H.D., Buzdar A., McGale P., Bonnefoi H., Colleoni M., Denkert C., Eiermann W., Jackesz R., Makris A., Miller W., Pierga J.-Y., Semiglazov V., Schneeweiss A., Souchon R., Stearns V., Untch M., Loibl S.. Annals of Oncology.2007;18(12). CrossRef
- Current and emerging biomarkers in breast cancer: prognosis and prediction Weigel Marion T, Dowsett Mitch. Endocrine-Related Cancer.2010;17(4). CrossRef
- Ki67, chemotherapy response, and prognosis in breast cancer patients receiving neoadjuvant treatment Fasching Peter A, Heusinger Katharina, Haeberle Lothar, Niklos Melitta, Hein Alexander, Bayer Christian M, Rauh Claudia, Schulz-Wendtland Ruediger, Bani Mayada R, Schrauder Michael, Kahmann Laura, Lux Michael P, Strehl Johanna D, Hartmann Arndt, Dimmler Arno, Beckmann Matthias W, Wachter David L. BMC Cancer.2011;11(1). CrossRef
- ER, PgR, Ki67, p27Kip1, and histological grade as predictors of pathological complete response in patients with HER2-positive breast cancer receiving neoadjuvant chemotherapy using taxanes followed by fluorouracil, epirubicin, and cyclophosphamide concomitant with trastuzumab Kurozumi Sasagu, Inoue Kenichi, Takei Hiroyuki, Matsumoto Hiroshi, Kurosumi Masafumi, Horiguchi Jun, Takeyoshi Izumi, Oyama Tetsunari. BMC Cancer.2015;15(1). CrossRef
- Primary chemotherapy in breast invasive carcinoma: predictive value of the immunohistochemical detection of hormonal receptors, p53, c-erbB-2, MiB1, pS2 and GST pi MacGrogan G, Mauriac L, Durand M, Bonichon F, Trojani M, de Mascarel I, Coindre JM. British Journal of Cancer.1996;74(9). CrossRef
- Clinical Course of Breast Cancer Patients With Complete Pathologic Primary Tumor and Axillary Lymph Node Response to Doxorubicin-Based Neoadjuvant Chemotherapy Kuerer Henry M., Newman Lisa A., Smith Terry L., Ames Fred C., Hunt Kelly K., Dhingra Kapil, Theriault Richard L., Singh Gurpreet, Binkley Susan M., Sneige Nour, Buchholz Thomas A., Ross Merrick I., McNeese Marsha D., Buzdar Aman U., Hortobagyi Gabriel N., Singletary S. Eva. Journal of Clinical Oncology.1999;17(2). CrossRef
- Neoadjuvant Chemotherapy with Docetaxel, Carboplatin and Weekly Trastuzumab Is Active in HER2-Positive Early Breast Cancer: Results after a Median Follow-Up of over 4 Years Kolberg Hans-Christian, Akpolat-Basci Leyla, Stephanou Miltiades, Aktas Bahriye, Hannig Carla Verena, Liedtke Cornelia. Breast Care.2016;11(5). CrossRef
- P304 Docetaxel, carboplatin and weekly trastuzumab are active as neoadjuvant therapy in operable HER2-positive breast cancer Kolberg H-C, Akpolat-Basci L, Otterbach F, Drumm A, Tirier C. The Breast.2011;20:S74.
- Pathologic Complete Response After Neoadjuvant Chemotherapy Plus Trastuzumab Predicts Favorable Survival in Human Epidermal Growth Factor Receptor 2–Overexpressing Breast Cancer: Results From the TECHNO Trial of the AGO and GBG Study Groups Untch Michael, Fasching Peter A., Konecny Gottfried E., Hasmüller Stephan, Lebeau Annette, Kreienberg Rolf, Camara Oumar, Müller Volkmar, du Bois Andreas, Kühn Thorsten, Stickeler Elmar, Harbeck Nadia, Höss Cornelia, Kahlert Steffen, Beck Thomas, Fett Werner, Mehta Keyur M., von Minckwitz Gunter, Loibl Sibylle. Journal of Clinical Oncology.2011;29(25). CrossRef
- Retrospective study of efficacy and safety of neoadjuvant docetaxel, carboplatin, and trastuzumab in HER2-positive locally advanced and oligometastatic breast cancer: An Indian experience Gogia A, Tiwari A, Deo SVS, Shukla NK, Mathur S, Sharma DN. Indian Journal of Cancer.2017;54(1). CrossRef
- Efficacy of neoadjuvant therapy with trastuzumab concurrent with anthracycline- and nonanthracycline-based regimens for HER2-positive breast cancer Bayraktar Soley, Gonzalez-Angulo Ana M., Lei Xiudong, Buzdar Aman U., Valero Vicente, Melhem-Bertrandt Amal, Kuerer Henry M., Hortobagyi Gabriel N., Sahin Aysegul A., Meric-Bernstam Funda. Cancer.2011;118(9). CrossRef
- Lapatinib with trastuzumab for HER2-positive early breast cancer (NeoALTTO): a randomised, open-label, multicentre, phase 3 trial Baselga José, Bradbury Ian, Eidtmann Holger, Di Cosimo Serena, de Azambuja Evandro, Aura Claudia, Gómez Henry, Dinh Phuong, Fauria Karine, Van Dooren Veerle, Aktan Gursel, Goldhirsch Aron, Chang Tsai-Wang, Horváth Zsolt, Coccia-Portugal Maria, Domont Julien, Tseng Ling-Min, Kunz Georg, Sohn Joo Hyuk, Semiglazov Vladimir, Lerzo Guillermo, Palacova Marketa, Probachai Volodymyr, Pusztai Lajos, Untch Michael, Gelber Richard D, Piccart-Gebhart Martine. The Lancet.2012;379(9816). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2021
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times