Cigarettes Smoking Pattern in Patient with Urothelial Carcinoma of the Bladder after Smoking Cessation in Tertiary Hospital
Download
Abstract
Background: Urothelial carcinoma is the most widely recognized histologic type of bladder cancer. Smoking duration and intensity associated with tumour aggressiveness and recurrence. This study aims to examine smoking behavioural change in patients with bladder urothelial carcinoma who were given an intervention for smoking cessation.
Methods: A cross-sectional study (2009 – 2019) was conducted. Subjects were divided into three groups: patients who had smoked <20 cigarettes per day for ≤30 years; patients who had smoked for 31–40 years or had smoked >20 cigarettes per day for ≤30 years; and patients who had smoked for >40 years. General characteristics, educational level and quit levels are noted. These data were obtained from the medical record and anamnesis by telephone.
Result: A total of 325 patients with urothelial cancer between 2009-2019 were involved. 273 (84%) patients were male, aged 58.71 ± 12.8 years. 40.9% had a history of smoking for >40 years, 66.5% of patients did not quit smoking at all. There was a significant difference in smoking cessation rates between groups with a history of different smoking intensities (p = 0.000). Mann-Whitney U test showed that there was a significant difference between the group who had a history of smoking <20 cigarettes for ≤30 years and the group who smoked for 31-40 years or >20 cigarettes per day for ≤30 years with the group who smoked for >40 years (p = 0.000 on both comparisons). 135 (41.5%) patients were in the lower education group.
Conclusion: The patient’s history of heavy smoking intensity and lower educational level was associated with a lower likelihood of smoking cessation. Urologists must play an important role in the education and intervention process to support patients to quit smoking. A guide/guideline is needed and it could be through a special smoking clinic for counselling or treatment.
Introduction
According to GLOBOCAN 2020, the incidence of bladder cancer is 573,278 for both sexes of all ages, making it the tenth leading cancer globally. Asia holds the highest incidence and mortality rate for bladder cancer among other regions, 36.3% and 42.6% respectively. There were 18,911 new cases and 10,327 deaths of bladder cancer in southeast Asia in 2020 [1]. The incidence rate of bladder cancer in Indonesia is on the fourteenth rank (2.0%) amongst other types of cancer, with a 1.7% mortality rate [2]. Based on data from the Indonesian Ministry of Health sourced from the Dharmais Cancer Hospital in 2018, bladder cancer is ranked 8th as the most common cancer in men with a proportion of 4.2% [3]. The study by Perix et al [4] reported the incidence rate of bladder cancer ranged from 1.41% - 2.71% in 2010-2014 at Hasan Sadikin General Hospital, Bandung, West Java [4].
The incidence of bladder cancer is twice as high in developing countries compared to developed countries. Urothelial carcinoma is the most widely recognized histologic type of bladder cancer (around 90%). Most bladder cancers are squamous cell carcinoma in most developing countries [5].
In 2015, WHO estimates that there were 1143 million smokers all around the world. Cigarette smoke is known to be the most common carcinogenic substance for humans [6]. Tobacco contains in more than 60 cancer-causing agents, causes at any rate 18 sorts of malignancy, and is the subsequent driving danger factor for death [7]. Tobacco smoking is the major risk factor for bladder cancer in both men and women, which account for >50% of bladder cancer cases [6]. Because of increasing tobacco use, intervention for smoking cessation has become an urgent need, especially in developing countries. Counselling and behavioural management are important to achieve smoking cessation. The ‘5 A’s-based intervention in the form of Ask, Advise, Assess, Assist and Arrange is also important to be implemented by a physician [7, 8].
There is a pathophysiological pathway between smoking and the incidence of bladder cancer. Tobacco smoke contains aromatic amines and polycyclic aromatic hydrocarbons that are known to have the ability to cause malignancy. These substances are excreted by the kidney and may exert carcinogenic effects on the urinary tract. The summarized findings from some studies suggest a substantial increase in the risk of urinary tract cancer for cigarette smokers. Duration of smoking and the number of cigarettes smoked was positively associated with urinary tract cancer risk [6, 9].
Addiction is a strong attachment that makes the person experience the difficulty to avoid the activity even when they know it causes harm [10]. Smoking causes the person to inhale nicotine, which enters the circulation rapidly through the lungs and moves into the brain within seconds. Rapid administration of nicotine potentiates locomotor sensitization, linked to a reward mechanism, and neuroplastic changes in the brain. The smoking process also provides rapid reinforcement and allows for precise dosing, making it possible for a smoker to obtain desired effects without toxicity. In addition to delivering nicotine to the brain quickly, cigarettes have been designed with additives and engineering features to enhance their addictiveness [11].
The accumulation of nicotine in the body is achieved within 6 to 9 hours of regular smoking and results in 24 hours of exposure. Arteriovenous differences in nicotine concentrations during cigarette smoking are substantial, with arterial levels might be up to 10 times as high as venous levels. The persistence of nicotine in the brain throughout the day and night changes the structure and function of nicotinic receptors and stimulating intracellular processes of neuroadaptation [11].
Smokers tend to take in the same amount of nicotine from day to day to achieve the desired effects. They adjust their smoking behaviour to compensate for changes in the availability of nicotine to regulate the body’s level of nicotine. Light smokers (those who smoke ≤5 cigarettes per day) and occasional smokers smoke primarily for the positive reinforcing effects of nicotine and have minimal or no withdrawal symptoms. Although withdrawal symptoms may not be prominent, many light and occasional smokers have difficulty quitting. Some of them have a high level of dependence, but with pharmacodynamics that differs from those in heavier smokers [11].
To achieve the cessation of tobacco consumption, behavioural and pharmacotherapy can be done. Even among persons who might ultimately achieve tobacco abstinence without therapy, the benefits can be profound if the treatments help people to achieve tobacco abstinence earlier because the risk of disease is strongly related to the duration of tobacco use. It can be in any form, which includes the physician giving advice to their patient to quit smoking [7].
Simple advice from a physician has been shown to increase abstinence rates significantly compared to no advice. United States guidelines recommend the “Five A’s approach”: ask about tobacco use; advise all users to quit; assess willingness to make a quit attempt; assist the patient to quit; and, arrange follow-up contact. Whereas the absolute effect of brief advice is relatively small, this intervention can have a considerable global impact because of the large number of people who visit their physicians. The physician can offer strong support, help set a quitting date, prescribe pharmaceutical therapies for nicotine dependence, such as replacement therapy and/or bupropion (with instructions for use), and suggest behavioural strategies to prevent relapse [7].
According to a 2015 survey, about 70% of current adult smokers in the United States wanted to quit, and although about 55% had attempted to do so in the past year, only 7% were successful in quitting for 6-12 months [12].
The typical assessment of the association between cancer occurrence and cigarette smoking usually using smoking duration and intensity, as measured by the number of cigarettes smoked per day. Duration of smoking is influenced by the differences in total exposure to cigarette smoke. To assess the effect of smoking intensity, one would typically compare the ORs of smoking for 30 years and smoking for 40 years among individuals who smoke 20 cigarettes per day to the ORs of smoking for 30 years and smoking for 40 years among individuals who smoke 30 cigarettes per day. Differences in the patterns of these ORs for duration would then be ascribed to the effects of intensity [13, 14].
The aim of this study is to identify the correlation of attempt of smoking cessation to smoking intensity and level of education in patients diagnosed with urothelial cell carcinoma.
Materials and Methods
A cross-sectional study (2009 – 2019) was conducted at Hasan Sadikin Academic Medical Center, a tertiary hospital in Indonesia. We analyzed the correlation between smoking quit level and smoking intensity and education level. Subjects were divided into three groups: patients who had smoked <20 cigarettes per day for ≤ 30 years; patients who had smoked for 31–40 years or had smoked >20 cigarettes per day for ≤ 30 years; and patients who had smoked for >40 years. The educational status of the patients was divided into the lower (taking formal education for ≤6 years or equivalent to elementary school), middle (taking formal education for 7 - ≤12 years or equivalent to high school), and higher education group (formal education for >12 years).
The inclusion criteria of this study were all patients diagnosed with urothelial carcinoma who had a history of tobacco smoking and were given the advice to quit smoking from 2009 – 2019. The exclusion criteria were all patients with incomplete medical records and who had died before given advice to quit smoking. This study was approved by our Institutional Review Board of Hasan Sadikin Academic Medical Center and informed consent was obtained from the subjects.
The recorded demographic characteristics of the patients included age and gender. The smoking quit level status was divided into three: total quit; partial quit; and do not quit. These data were obtained from the medical records and also using a questionnaire that was asked by telephone.
The correlation was assessed between smoking quit level status with smoking intensity and educational level. Statistical comparison between the groups was performed using the Kruskal Wallis test, and post hoc Mann-Whitney U test. Statistical analysis was performed using the IBM SPSS Statistics software (IBM Corp., NY) version 24. A P-value of <0.05 was considered statistically significant.
Results
Information for a total of 325 patients with urothelial bladder disease somewhere in the range of 2009 and 2019 were gathered.
The demographic characteristics of the patients are shown in Table 1.
| Characteristics | All patients (n=325) | Patients smoked <20 cigarettes per day for ≤30 years (n=94) | Patients smoked for 31-40 years or had smoked >20 cigarettes per day for ≤30 years (n=98) | Patients smoked for > 40 years (n=133) |
| Sex | ||||
| ● Male | 273 (84%) | 73 (77,7%) | 84 (85,71%) | 116 (87,21%) |
| ● Female | 52 (16%) | 21 (22,3%) | 14 (14,29%) | 17 (12,79%) |
| Age (mean ± SD) | 58,71 ± 12,8 years | 54,99 ± 14,3 years | 55,91 ± 10,6 years | 63,41 ± 11,8 years |
| Education Level | ||||
| ● Lower | 135 (41,5%) | 40 (42,6%) | 40 (40,8%) | 55 (41,4%) |
| ● Middle | 131 (40,3%) | 40 (42,6%) | 39 (39,8%) | 52 (39,1%) |
| ● Higher | 59 (18,2%) | 14 (14,9%) | 19 (19,4%) | 26 (19,5%) |
| Quit Level | ||||
| ● Total quit | 52 (16,0%) | 30 (31,9%) | 13 (13,3%) | 9 (6,8%) |
| ● Partial quit | 57 (17,5%) | 14 (14,9%) | 27 (27,6%) | 16 (12,0%) |
| ● Do not quit | 216 (66,5%) | 50 (53,2%) | 58 (59,2%) | 108 (81,2%) |
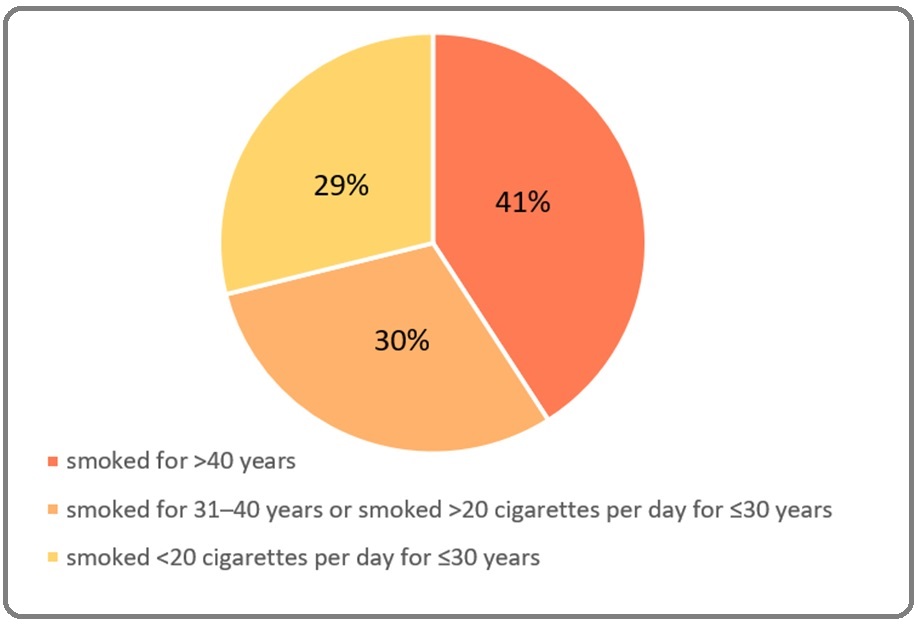
A total of 273 (84%) patients were male (Figure 1), with the mean population age of 58.71 ± 12.8 years. Of the 325 patients, most (40.9%) had a history of smoking for more than 40 years, followed by 30.2% who had smoked for 31-40 years or smoked >20 cigarettes for ≤20 years, and 28.9% of patients smoked for 20 years (Figure 2).
Figure 1. Gender Distribution.
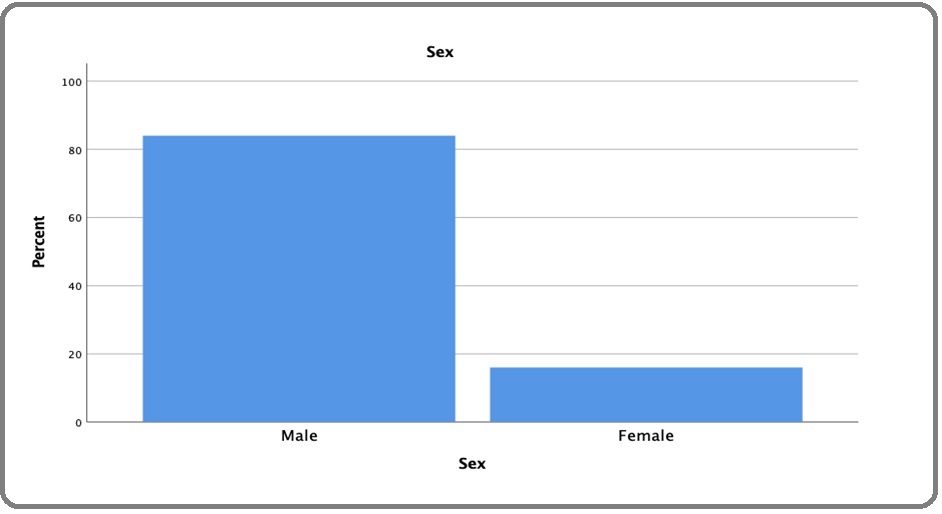
Figure 2. The Proportion of Smoking Iintensity Level.
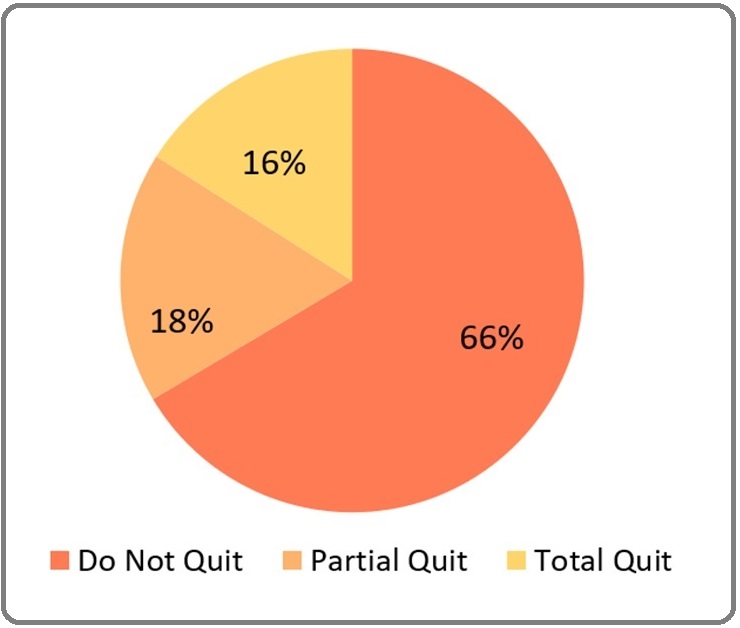
In this study, 66,5% of patients did not quit smoking at all, followed by 17,5% who quitted smoking partially and only 16,0% of patients who quitted smoking completely after diagnosed with bladder carcinoma and given smoking cessation interventions (Figure 3).
Figure 3. The Proportion of Smoking Quit Level.
The Kruskal Wallis test showed a significant difference in smoking cessation rates between groups with a history of different smoking intensities (p = 0.000) (Table 2).
| Characteristics | Total Quit (n=52) | Partial Quit (n=57) | Do Not Quit (n=216) | P value |
| Sex | 0,022 | |||
| ● Male | 37 (71,2%) | 49 (86,0%) | 187 (86,6%) | |
| ● Female | 15 (28,8%) | 8 (14,0%) | 29 (13,4%) | |
| Education Level | 0 | |||
| ● Lower | 10 (19.2%) | 23 (40,4%) | 102 (47,2%) | |
| ● Middle | 19 (36.5%) | 22 (38,6%) | 90 (41,7%) | |
| ● Higher | 23 (44,2) | 12 (21,1%) | 24 (11,1%) | |
| Smoking Intensity | 0,000 | |||
| Smoked <20 cigarettes per day for ≤30 years | 30 (31,9%) | 14 (14,9%) | 50 (53,2%) | |
| ● Smoked for 31–40 years or had smoked >20 cigarettes per day for ≤30 years | 13 (13,3%) | 27 (27,6%) | 58 (59,2%) | |
| ● Smoked for >40 years | 9 (6,8%) | 16 (12,0%) | 108 (81,2%) |
In the post hoc analysis using the Mann-Whitney U test, there was a significant difference between the group who had a history of smoking <20 cigarettes for ≤30 years and the group who smoked for 31-40 years or >20 cigarettes per day for ≤30 years with the group who smoked for >40 years (p = 0.000 on both comparison) (Figure 4).
Figure 4. The Proportion of Quit Level Based on Different Smoking Intensity Levels.
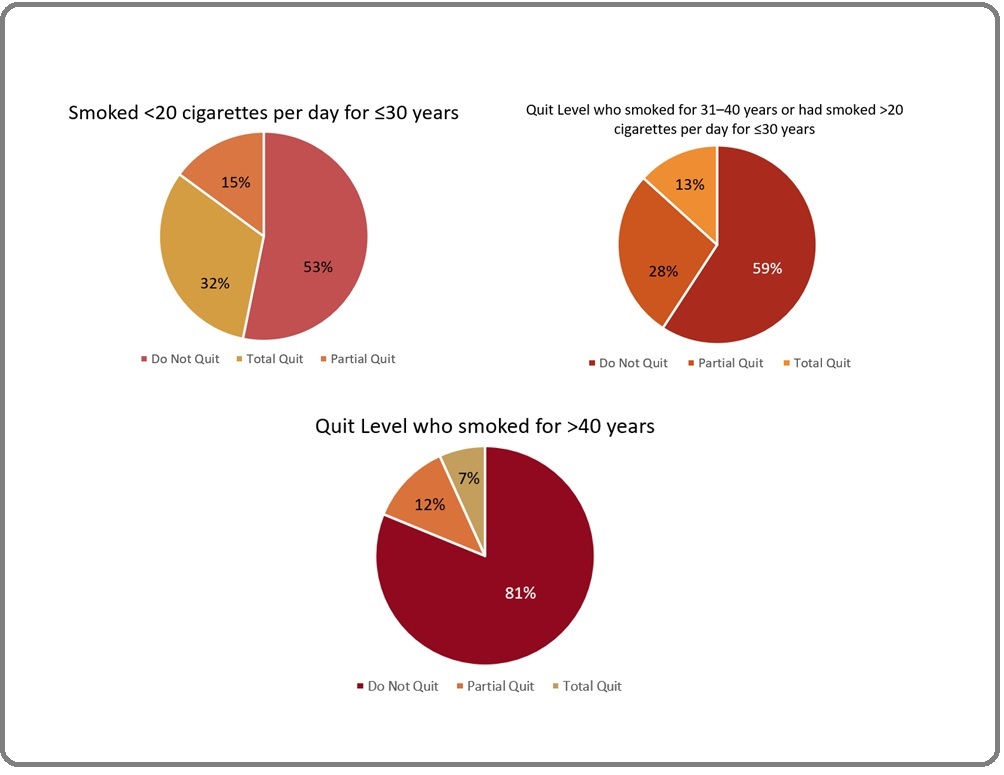
The group with a history of the heaviest smoking intensity was found to have significantly lower smoking cessation rates than the group with a history of lighter smoking (Table 3).
| Groups | Comparison Groups | P value |
| Smoked <20 cigarettes per day for ≤30 years | Smoked for 31–40 years or had smoked >20 cigarettes per day | 0.087 |
| Smoked for 31-40 years or had smoked >20 per day for ≤30 years | for ≤30 years | 0 |
| Smoked for >40 years | 0 |
For the characteristics of the patient’s educational level, it was found that 135 (41.5%) patients were included in the lower education group (formal education ≤6 years), 131 (40,3%) were in the middle education group (formal education for 7 - ≤12 years), and 59 (18,2%) patients had higher educational level (Figure 5).
Figure 5. The Proportion of Overall patient’s Educational Level and Quit Level Based on Different Educational levels.
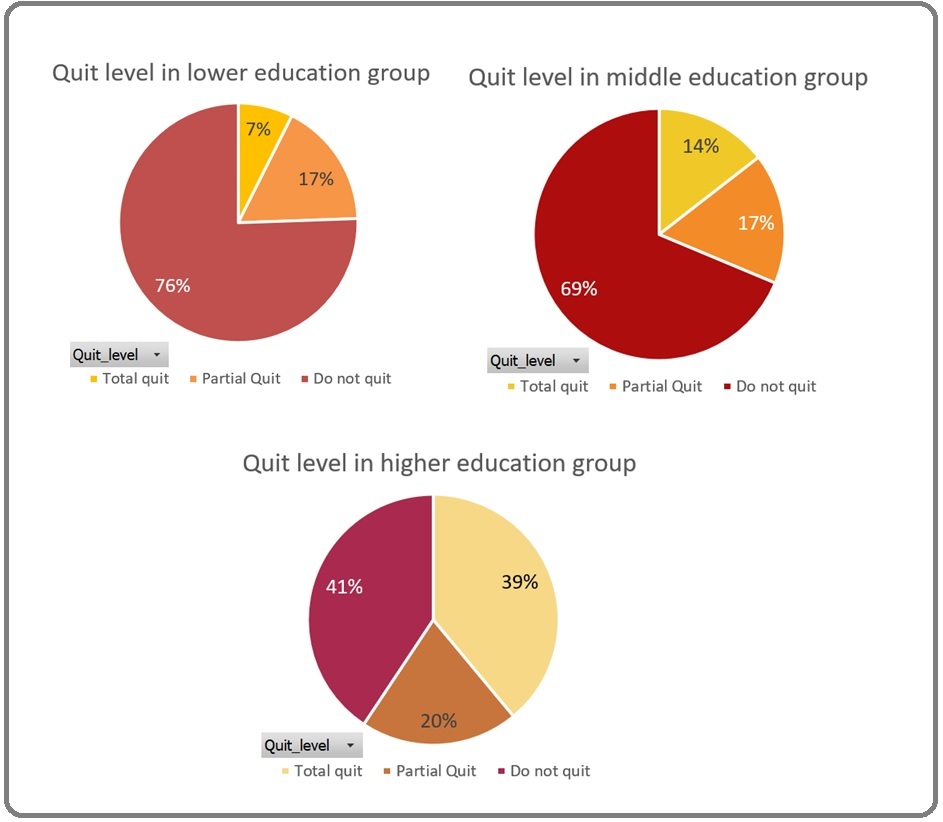
The Kruskal Wallis statistical test showed that there were significant differences between groups with different quit levels in terms of educational level (p = 0.000). Patient groups that did not quit smoking tended to be associated with lower levels of education. Patients with higher educational levels were associated with an increased chance to quit smoking (Table 2).
Discussion
Urothelial carcinoma is the most common histologic type of bladder cancer. It is the invasion of the basement membrane or lamina propria or deeper by neoplastic cells of the urothelial [5]. Important risk factors include smoking, schistosomiasis infection, and occupational exposure to certain chemicals. Smoking is the most important risk factor for bladder cancer. The risk of bladder cancer in smokers is 2 to 6 fold that of non-smokers; the risk depends on smoking duration and intensity. In developing countries, schistosomiasis infection is an important cause of bladder cancer. Occupational exposure to paint, rubber, petroleum products, and dyes correlate to bladder cancer. Chemicals associated with bladder cancer include arylamine dye, aniline dye, phenacetin, cyclophosphamide, and arsenic [5].
There are two distinct pathways of urothelial carcinoma. The first relates to papillary lesions, and the second relates to flat lesions. Low-grade papillary tumors usually arise from simple hyperplasia and/or minimal dysplasia and are characterized by loss of heterozygosity (LOH) of chromosome 9 and activating mutations of fibroblast growth factor receptor 3 (FGFR3), telomerase reverse transcriptase (TERT), phosphatidylinositol 4,5-bisphosphate 3-kinase catalytic subunit alpha isoform (PIK3CA) and inactivating mutations of STAG2. Low- grade papillary non-muscle-invasive bladder cancer can progress to muscle-invasive bladder cancer as a result of gaining CDKN2A loss. Muscle-invasive bladder cancer arises from flat dysplasia or carcinoma in situ (CIS); the lesions show TP53 mutations and LOH of chromosome 9. The invasive carcinoma can then further gain RB1 and PTEN loss along with other alterations acquiring metastatic potential [5].
Bladder cancer risk factors are classified into two categories: those that cannot be modified and those that can. Gender is an unchangeable risk factor for bladder cancer. Bladder cancer is said to be three times more common in men than it is in women, which is consistent with the results of this study which showed that up to 84% of patients diagnosed with urological cancer were male. Men are more likely to resort to smoking as a result of a higher proclivity for risky behaviors and exposure to a variety of pressures brought on by work, family, and social obligations [15, 16]. Smoking and the environment/workplace chemical exposure are two risk factors for bladder cancer that can be changed. Smoking is a significant risk factor for bladder cancer, accounting for 50% of cases [17]. Cigarettes contain hydrocarbons, aromatic amines, 2 naphtylamine, and N-nitroso, all of which can damage DNA by forming bulky adducts and disrupting single and multiple DNA chains, resulting in uncontrolled cell growth and inhibition of the tumor growth inhibitory mechanism. Xenobiotic enzymes, such as N-acetyltransferase (NAT) and glutathione S-transferase, metabolize carcinogens in cigarettes. Cigarette carcinogens are excreted in the urine, resulting in direct contact with the urinary tract, which raises the risk of cancer. There will be a decrease in the efficiency of carcinogen detoxification if anyone has a slow NAT2 acetylation activity (common in smokers), as carcinogens accumulate in high levels in the urothelium [18]. Previous studies have also found that smoking duration and the number of cigarettes smoked were also associated with an increased risk of urinary tract cancer [6, 15]. In this study, it was found that 40.9% of patients diagnosed with urological malignancy had a history of smoking for more than 40 years.
The period of time that a patient was diagnosed with cancer can be a “teachable moment” that can help encourage the patient to make lifestyle changes [19]. Study by Bassett et al [20]. found that patients newly diagnosed with bladder cancer were five times more likely to quit smoking when compared to smokers in the general population (48% vs. 10%, p <0.001) [20]. Even so, it is striking that only 16% of patients in this study who quitted smoking completely after being diagnosed with urothelial cancer and given smoking cessation interventions. This number is only slightly higher than that one reported in the study by van Osch et al. [21] where as many as 14% of patients quit smoking after being diagnosed with bladder cancer [21]. Majority of smokers around the world really want to quit, but this is not an easy task to do. In the United States, it is reported that more than 70% of smokers have tried at least 1 attempt to quit smoking, but only about 7% are successful for abstinence during the 1 year thereafter [22]. Despite the development of various intensive smoking cessation intervention strategies, the overall success rate of long-term smoking abstinence is still modest [23]. It often takes multiple quit efforts and persistence for smokers in cognitive and behavioral abilities to resist withdrawal symptoms, negative affect and strong urge or cravings [22]. The number of patients who quit smoking in this study was in concordance with the results that reported in previous meta-analysis by Zhao et al. [10] that involved 21 studies and 3,669 patients diagnosed with urological cancer, and found smoking cessation rates ranged from 14.3% to 79.0% [10]. The large variability in smoking cessation rates may be due to the heterogeneity of the study population, study design, and settings. The low proportion of patients who quit smoking in this study can be explained by several factors such as history of heavy smoking load, patients mostly diagnosed with non-invasive bladder cancer with significant cure and survival potential, suboptimal awareness of the importance of smoking in bladder cancer development, or suboptimal education and promotion of cessation by urologists [24].
Nevertheless, smoking cessation after urological cancer diagnosis has been consistently reported to be of great benefit to patients, where smoking cessation has been reported to be associated with improved treatment outcomes, reduced risk of disease recurrence, and prolonged survival when compared with patients who continued smoking after diagnosis [25]. Patients with bladder cancer who continued smoking have increased risk of a poorer outcome, poorer quality of life and the presence of secondary cancers [26]. One retrospective cohort study by Chen et al. [27] reported that smoking cessation after bladder cancer diagnosis significantly reduced the risk of recurrence (HR 0.45, 95% CI 0.25-0.83) when compared with patients who continued smoking [27]. Moreover, considering the impact of comorbidities including the various diseases associated with smoking on overall survival in patients with bladder cancer, smoking cessation should be encouraged once the patient is diagnosed with bladder cancer [21]. Therefore, smoking cessation education and therapy program still need to be integrated into the routine care of patients with urological cancer at this time of teachable moment in order to support patients’ efforts to quit smoking and would improve treatment outcomes [10].
In this study it was found that patients with a history of the heaviest smoking intensity had a lower tendency to quit smoking compared to patients with lighter smoking intensity. Previous research has concluded that light-daily smokers are more likely to have been abstinent for at least a year, are more likely to attempt to quit, and are more likely to succeed in their attempt, and light-intermittent smokers are still more likely to successfully quit [28, 29]. Analysis by Ylioja et al. [30] also previously demonstrated that light smokers were significantly more likely to quit smoking after counseling and pharmacotherapy interventions.[30] Research by Lee et al. [31] proved that higher number of cigarette consumption per day was associated with lower abstinence rate at follow-up [31]. A recent study by Ni et al. [32] reported that, although grouping smokers into groups based on the level of cigarette consumption was not independently associated with smoking cessation rates, the history of smoking intensity could influence their confidence level to quit, which is an important predictor of smoking cessation [32]. The level of cigarette smoking increases as the smoking time lengthens, and the higher the amount of cigarette smoking, the stronger the nicotine dependency. As a result, early smoking interventions must be given as soon as possible before the addiction progresses to a more serious stage [31]. Based on these observations, type of smoker based on intensity may be a useful predictor of quitting likelihood, and specialized programs targeted at heavy smokers may be needed to facilitate better withdrawal for these individuals. During the first weeks of abstinence, smokers in this group can benefit from more intensive pharmacological treatment for smoking cessation [33].
In this study, it was found that most of the patients diagnosed with urological cancer and had a history of smoking had a lower and middle level of education, which was defined by formal education ≤12 years or equivalent to high school. This is consistent with the findings of previous studies which demonstrated a higher prevalence of smoking in patients with low level of education, compared to those with high education. [34] Education, a measure of socioeconomic status, has been shown to be inversely related to cancer incidence, especially for smoking-related cancers [35-38]. Lower levels of education were found to be common in patients with urologic cancer, as reported by Westhoff et al. [39] in a study where up to 77% of patients with bladder cancer had a low level of education. The study also showed that patients with a low level of education were less likely to be aware of the causal factors that could cause urological cancer (32% versus 19% in patients with a high level of education), such as smoking [39]. Educational level of is also known to correlate closely with smoking status which is the most important risk factor for bladder cancer [40]. This study also demonstrated that patients with lower levels of education are more likely to have difficulty in quitting smoking, which is consistent with the results of previous studies by Gilman et al. [41], Lillard et al. [42], Reid et al. [43], Wetter et al. [44], and Zhuang et al. [45] [41-45]. However, the current study cannot explain why this difference exists. One possible explanation is that smokers with a lower level of education are more likely to live in an environment where cigarettes are more accessible than smokers with higher education [46]. As a result, they are more likely to be surrounded by family, friends, or coworkers who are also smokers and are more likely to view smoking as a common thing [47]. Another possible explanation is that smokers with lower educational level tend to have less financial resources and psychological support to support their efforts to quit smoking than those with higher educational level [29,48]. Smokers with low levels of education also have poor health literacy and comprehension abilities, necessitating more regular and comprehensive psychological assistance in order to quit smoking [49].
However, inconsistencies in results were also found by previous studies. Several other studies showed that smoking cessation rates did not differ significantly between education level groups, and suggest that once a person becomes a smoker, it will be difficult for them to quit smoking regardless of their level of education [50, 51]. One study even showed an inverse relationship between education level and smoking cessation rates, whereby smokers with lower levels of education were more likely to succeed in smoking cessation efforts [52]. The findings could be due to the assumption that smokers with a high level of education tend to include groups of individual workers with higher income. They are faced with a higher level of work stress, and with sufficient financial and social environment encouragement it is easier to smoke and difficult to quit [49]. The inconsistencies in these reports could be due to the heterogeneity in the type and size of the sample and the analytical approach used [49].
In this study, 17,5% of patients quit smoking partially, and up to 66,5% of patients did not quit smoking at all. It is important for the patients to be aware of the connection between smoking and its effects in order to change behavior, as it increases the patient’s desire to quit [53]. The lack of knowledge and awareness about the relationship between cigarette smoke exposure and the urological cancer carcinogenesis process in the general population might be one of the factors that causes patients not to stop smoking even though they have been diagnosed with urological cancer. A cross-sectional study found that only 25-36% of patients were aware that smoking was a risk factor for bladder cancer, compared with 94-98% who were aware of patients with lung cancer [54]. Given the important role of exposure to smoking in the progression and natural history of many urological diseases, especially urogenital carcinoma, and taking into account of the patient-urologist relationships and interactions, urologists should play an important role in smoking cessation education and interventions process in patients diagnosed with urological cancer [55]. Previous studies found that apart from a diagnosis of cancer, advice from urologists was also the most motivated reason for smoking cessation, cited by 55% patients, versus 28% cited the advice of the primary care physician [20]. Therefore, any active or ex-smoker encountered in daily clinical practice, regardless of their level of dependence, should receive a personalized and explicit educational message from their urologist regarding the toxicity effects of smoking and its effect on urological disease experienced by patients [55]. In view of the above, urologists continue to play a critical role. According to Bjurlin et al. [56], a single 5-minute brief smoking cessation intervention provided by a urologist was linked to a higher rate of cessation attempt by patients [56]. Several public health institutions have established and approved a so-called five A’s intervention, which stands for Ask, Advice, Assess, Assist, and Arrange. “Ask” implies that any patient seen by a urologist should be asked whether or not they smoke. “Advice” includes both authoritatively urging the patient to quit smoking and a brief overview of the benefits of quitting smoking in terms of both urologic and general health. The patient then need to “Assess” if the patient is going to try to stop smoking right now. “Assist” entails either initial medical or behavioral intervention, or at the very least a referral to a smoking cessation clinic. Finally, “Arrange” implies that the urologist should actively be assisting with follow-up or referral arrangements. The fifth A emphasizes the urologist’s involvement in the smoking cessation monitoring process [57].
This study has several limitations. The study design which is a cross-sectional study has its own limitations and limits conclusions about the causal relationship between variables in this study. Data limitations also prevented investigators from fully investigating the type of smoking cessation intervention provided, the setting when the intervention was given, and the detail of the time point at which smoking cessation data was taken is also unknown. The absence of controls also makes it difficult to distinguish between the likelihood of patients quitting smoking when compared with the general population. Due to the limited availability of data in this study, the variables that can be analyzed are also limited and make it difficult for researchers to control the effect of unobserved potential confounders.
In conclusions, this study demonstrated a sizeable proportion of patients who continued smoking after being diagnosed with urothelial carcinoma of bladder and given smoking cessation interventions. The patient’s heavy smoking intensity was associated with a lower likelihood of smoking cessation. Patients with lower levels of education tend to have more difficulty in quitting smoking. Urologists must play an important role in education and hospital must provide more intervention options to support patients to quit smoking. Seeing that there are still many patients who do not quit smoking, a guide / guideline is needed and it could be through a special smoking clinic for counselling or treatment to help patients quit smoking, which needs to be available in a health center such as a hospital or clinic.
References
- World Health Organization. The Global Cancer Observatory Bl cancer Glob fact sheet.2021.
- World Health Organization. The Global Cancer Observatory Indones fact sheet.2021.
- Kementerian Kesehatan Republik Indonesia. Beban Kanker di Indonesia Pus Data dan Inf Kemeterian Kesehat RI.2019;:1-16.
- Five Years Facts of Bladder Cancer at West Java’s Top Referral Hospital, in Indonesia Perix VK, Suryanti S, Sihombing AT. Althea Med J.2017;4(1):94-99.
- Bladder Cancer. (2021). In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2022 Jan.2021 Oct 30 Kaseb H, Aeddula N. .
- Smoking duration and intensity associated with tumour aggressiveness in patients with urothelial carcinoma of the bladder: A correlation study Pramod Sawkar Vijay, Safriadi Ferry, Hernowo Bethy S., Dwiyana Reiva Farah, Bonar Ananta. Journal of Clinical Urology.2021;14(5). CrossRef
- Smoking behavior and survival outcomes in bladder cancer patients Chu Wei-Chung, Chen Chung-Hsin. Urological Science.2020;31(3). CrossRef
- World Health Organization. Treatment of Tobacco Dependence and Smoking Cessation Methods 2002.
- Association between smoking and risk of bladder cancer among men and women Freedman Neal D., Silverman Debra T., Hollenbeck Albert R., Schatzkin Arthur, Abnet Christian C.. JAMA.2011;306(7). CrossRef
- A Systematic Review and Scoping Analysis of Smoking Cessation after a Urological Cancer Diagnosis Zhao Calvin, Bjurlin Marc A., Roberts Timothy, Rink Michael, Shariat Shahrokh F., Matulewicz Richard S.. The Journal of Urology.2021;205(5). CrossRef
- Addiction, cigarette smoking, and voluntary control of action: Do cigarette smokers lose their free will? Baumeister Roy F.. Addictive Behaviors Reports.2017;5. CrossRef
- Nicotine addiction Benowitz Neal L.. The New England Journal of Medicine.2010;362(24). CrossRef
- Brief opportunistic smoking cessation interventions: a systematic review and meta-analysis to compare advice to quit and offer of assistance Aveyard Paul, Begh Rachna, Parsons Amanda, West Robert. Addiction (Abingdon, England).2012;107(6). CrossRef
- Heme oxygenase-1 expression is associated with tumor aggressiveness and outcomes in patients with bladder cancer: a correlation with smoking intensity Miyata Yasuyoshi, Kanda Shigeru, Mitsunari Kensuke, Asai Akihiro, Sakai Hideki. Translational Research: The Journal of Laboratory and Clinical Medicine.2014;164(6). CrossRef
- Smoking history, smoking intensity, and type of cigarette as risk factors of bladder cancer: A literature review Pramod Sawkar Vijay, Safriadi Ferry, Hernowo Bethy S., Dwiyana Reiva Farah, Batista Baskara. Urological Science.2020;31(4). CrossRef
- Pattern of cigarette smoking: intensity, cessation, and age of beginning: evidence from a cohort study in West of Iran Hamzeh Behrooz, Farnia Vahid, Moradinazar Mehdi, Pasdar Yahya, Shakiba Ebrahim, Najafi Farid, Alikhani Mostafa. Substance Abuse Treatment, Prevention, and Policy.2020;15(1). CrossRef
- Urologic Malignancies Thuener Jennifer E.. Primary Care.2019;46(2). CrossRef
- The Role of Tobacco Smoke in Bladder and Kidney Carcinogenesis: A Comparison of Exposures and Meta-analysis of Incidence and Mortality Risks Cumberbatch Marcus G., Rota Matteo, Catto James W. F., La Vecchia Carlo. European Urology.2016;70(3). CrossRef
- The diagnosis of bladder cancer: are we missing a teachable moment for smoking cessation? Sosnowski Roman, Kamecki Hubert, Bjurlin Marc A., Przewoźniak Krzysztof. Translational Andrology and Urology.2019;8(Suppl 3). CrossRef
- Impact of a bladder cancer diagnosis on smoking behavior Bassett Jeffrey C., Gore John L., Chi Amanda C., Kwan Lorna, McCarthy William, Chamie Karim, Saigal Christopher S.. Journal of Clinical Oncology: Official Journal of the American Society of Clinical Oncology.2012;30(15). CrossRef
- The association between smoking cessation before and after diagnosis and non-muscle-invasive bladder cancer recurrence: a prospective cohort study Osch Frits H. M., Jochems Sylvia H. J., Reulen Raoul C., Pirrie Sarah J., Nekeman Duncan, Wesselius Anke, James Nicholas D., Wallace D. Michael A., Cheng K. K., Schooten Frederik J., Bryan Richard T., Zeegers Maurice P.. Cancer Causes & Control.2018;29(7). CrossRef
- Success rates in smoking cessation: Psychological preparation plays a critical role and interacts with other factors such as psychoactive substances Joly Bertrand, Perriot Jean, Athis Philippe, Chazard Emmanuel, Brousse Georges, Quantin Catherine. PloS One.2017;12(10). CrossRef
- Effectiveness of interventions to help people stop smoking: findings from the Cochrane Library Lancaster T., Stead L., Silagy C., Sowden A.. BMJ (Clinical research ed.).2000;321(7257). CrossRef
- Cigarette smoking patterns in patients after treatment of bladder cancer Ostroff J., Garland J., Moadel A., Fleshner N., Hay J., Cramer L., Zauber A., Trambert R., O'Sullivan M. E., Russo P.. Journal of Cancer Education: The Official Journal of the American Association for Cancer Education.2000;15(2). CrossRef
- Epidemiology and risk factors of urothelial bladder cancer Burger Maximilian, Catto James W. F., Dalbagni Guido, Grossman H. Barton, Herr Harry, Karakiewicz Pierre, Kassouf Wassim, Kiemeney Lambertus A., La Vecchia Carlo, Shariat Shahrokh, Lotan Yair. European Urology.2013;63(2). CrossRef
- [Smoking Cessation after Bladder Cancer Diagnosis] Mota Paulo, Sousa Pedro Miguel, Botelho Francisco, Carvalho-Dias Emanuel, Cordeiro Agostinho, Torres João Pimentel, Morais Nuno, Anacleto Sara, Lima Estevão. Acta Medica Portuguesa.2018;31(2). CrossRef
- Stopping smoking might reduce tumour recurrence in nonmuscle-invasive bladder cancer Chen Chung-Hsin, Shun Chia-Tung, Huang Kuo-How, Huang Chao-Yuan, Tsai Yu-Chieh, Yu Hong-Jeng, Pu Yeong-Shiau. BJU international.2007;100(2). CrossRef
- Level of Cigarette Consumption and Quit Behavior in a Population of Low-Intensity Smokers – Longitudinal Results from the International Tobacco Control (ITC) Survey in Mexico Swayampakala Kamala, Thrasher James, Carpenter Matthew J., Shigematsu Luz Myriam Reynales, Cupertio Ana-Paula, Berg Carla J.. Addictive behaviors.2013;38(4). CrossRef
- Changes in cigarette smoking initiation, cessation, and relapse among U.S. adults: a comparison of two longitudinal samples Yi Zinan, Mayorga Maria E., Hassmiller Lich Kristen, Pearson Jennifer L.. Tobacco Induced Diseases.2017;15. CrossRef
- Postdischarge smoking cessation in subgroups of hospitalized smokers: A latent class analysis Ylioja Thomas, Cochran Gerald, Chang Yuchiao, Tindle Hilary A., Rigotti Nancy A.. Substance Abuse.2017;38(4). CrossRef
- Predictors of Abstinence from Smoking: A Retrospective Study of Male College Students Enrolled in a Smoking Cessation Service Lee Yeji, Lee Kang-Sook, Kim Haena. International Journal of Environmental Research and Public Health.2019;16(18). CrossRef
- Does Smoking Intensity Predict Cessation Rates? A Study of Light-Intermittent, Light-Daily, and Heavy Smokers Enrolled in Two Telephone-Based Counseling Interventions Ni Katherine, Wang Binhuan, Link Alissa R., Sherman Scott E.. Nicotine & Tobacco Research: Official Journal of the Society for Research on Nicotine and Tobacco.2020;22(3). CrossRef
- Common predictors of smoking cessation in clinical practice Caponnetto Pasquale, Polosa Riccardo. Respiratory Medicine.2008;102(8). CrossRef
- Disparity in Smoking Prevalence by Education: Can We Reduce It? Zhu Shu-Hong, Hebert Kiandra K., Wong Shiushing, Cummins Sharon E., Gamst Anthony. Global health promotion.2010;17(1 Suppl). CrossRef
- Level of education and the risk of cancer in Sweden Hemminki K, Li X. Cancer Epidemiol Biomarkers Prev.2003;12(8):796-802.
- Behaviour partly explains educational differences in cancer incidence in the south-eastern Netherlands: the longitudinal GLOBE study Louwman W. J., Lenthe F. J., Coebergh J. W. W., Mackenbach J. P.. European journal of cancer prevention: the official journal of the European Cancer Prevention Organisation (ECP).2004;13(2). CrossRef
- Explaining the socioeconomic variation in cancer risk in the Norwegian Women and Cancer Study Braaten Tonje, Weiderpass Elisabete, Kumle Merethe, Lund Eiliv. Cancer Epidemiology, Biomarkers & Prevention: A Publication of the American Association for Cancer Research, Cosponsored by the American Society of Preventive Oncology.2005;14(11 Pt 1). CrossRef
- Education and risk of cancer in a large cohort of men and women in the United States Mouw Traci, Koster Annemarie, Wright Margaret E., Blank Madeleine M., Moore Steven C., Hollenbeck Albert, Schatzkin Arthur. PloS One.2008;3(11). CrossRef
- Low awareness of risk factors among bladder cancer survivors: New evidence and a literature overview Westhoff Ellen, Oliveira-Neumayer Julia Maria, Aben Katja K., Vrieling Alina, Kiemeney Lambertus A.. European Journal of Cancer.2016;60. CrossRef
- Occupational Exposure and Risk of Bladder Cancer Population based studies in the Nordic countries and Canada Hadkhale K. Tampere University Press.2018.
- Socioeconomic status over the life course and stages of cigarette use: initiation, regular use, and cessation Gilman S, Abrams D, Buka S. Journal of Epidemiology and Community Health.2003;57(10). CrossRef
- Who kicks the habit and how they do it: socioeconomic differences across methods of quitting smoking in the USA Lillard Dean R., Plassmann Vandana, Kenkel Donald, Mathios Alan. Social Science & Medicine (1982).2007;64(12). CrossRef
- Socioeconomic disparities in quit intentions, quit attempts, and smoking abstinence among smokers in four western countries: Findings from the International Tobacco Control Four Country Survey Reid Jessica L., Hammond David, Boudreau Christian, Fong Geoffrey T., Siahpush Mohammad. Nicotine & Tobacco Research.2010;12(Suppl 1). CrossRef
- What accounts for the association of education and smoking cessation? Wetter David W., Cofta-Gunn Ludmila, Irvin Jennifer E., Fouladi Rachel T., Wright Kelli, Daza Patricia, Mazas Carlos, Cinciripini Paul M., Gritz Ellen R.. Preventive Medicine.2005;40(4). CrossRef
- Comparison of Smoking Cessation Between Education Groups: Findings From 2 US National Surveys Over 2 Decades Zhuang Yue-Lin, Gamst Anthony C., Cummins Sharon E., Wolfson Tanya, Zhu Shu-Hong. American Journal of Public Health.2015;105(2). CrossRef
- Neighborhood smoking norms modify the relation between collective efficacy and smoking behavior Ahern Jennifer, Galea Sandro, Hubbard Alan, Syme S. Leonard. Drug and alcohol dependence.2009;100(1-2). CrossRef
- The collective dynamics of smoking in a large social network Christakis Nicholas A., Fowler James H.. The New England Journal of Medicine.2008;358(21). CrossRef
- What accounts for the relationship between social class and smoking cessation? Results of a path analysis Honjo Kaori, Tsutsumi Akizumi, Kawachi Ichiro, Kawakami Norito. Social Science & Medicine (1982).2006;62(2). CrossRef
- Relationship between education levels and booster counselling sessions on smoking cessation among Chinese smokers Wu Lei, He Yao, Jiang Bin, Zuo Fang, Liu Qinghui, Zhang Li, Zhou Changxi, Liu Miao, Chen Hongyan. BMJ Open.2015;5(8). CrossRef
- Predictors of smoking cessation in a cohort of adult smokers followed for five years Hymowitz N., Cummings K. M., Hyland A., Lynn W. R., Pechacek T. F., Hartwell T. D.. Tobacco Control.1997;6 Suppl 2. CrossRef
- Attempts to quit smoking and relapse: factors associated with success or failure from the ATTEMPT cohort study Zhou Xiaolei, Nonnemaker James, Sherrill Beth, Gilsenan Alicia W., Coste Florence, West Robert. Addictive Behaviors.2009;34(4). CrossRef
- Predictors of quit attempts and successful quit attempts in a nationally representative sample of smokers Rafful Claudia, García-Rodríguez Olaya, Wang Shuai, Secades-Villa Roberto, Martínez-Ortega Jose M., Blanco Carlos. Addictive Behaviors.2013;38(4). CrossRef
- Teachable moments for promoting smoking cessation: the context of cancer care and survivorship McBride Colleen M., Ostroff Jamie S.. Cancer Control: Journal of the Moffitt Cancer Center.2003;10(4). CrossRef
- Are patients aware of the association between smoking and bladder cancer? Nieder Alan M., John Seeniann, Messina Catherine R., Granek Iris A., Adler Howard L.. The Journal of Urology.2006;176(6 Pt 1). CrossRef
- Role of cigarette smoking in urological malignancies and clinical interventions for smoking cessation Sosnowski Roman, Bjurlin Marc A., Verze Paolo, De Nunzio Cosimo, Shariat Shahrokh F., Brausi Maurizio, Donin Nicholas M.. Central European Journal of Urology.2016;69(4). CrossRef
- Brief smoking cessation intervention: a prospective trial in the urology setting Bjurlin Marc A., Cohn Matthew R., Kim Dae Y., Freeman Vincent L., Lombardo Lindsay, Hurley Stephen D., Hollowell Courtney M. P.. The Journal of Urology.2013;189(5). CrossRef
- Effectiveness of the 5-As Tobacco Cessation Treatments in Nine HMOs Quinn Virginia P., Hollis Jack F., Smith K. Sabina, Rigotti Nancy A., Solberg Leif I., Hu Weiming, Stevens Victor J.. Journal of General Internal Medicine.2009;24(2). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2022
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times