Prevalence of H. pylori Infection in Relatives of Peruvian Patients with Gastric Cancer
Download
Abstract
Objective: The aim of this study was to determine the relationship between Helicobacter pylori (H. pylori) infection in relatives and patients with gastric cancer (GC).
Methods: H. pylori infection was evaluated by the breath urease test in 171 relatives and by qPCR technique in gastric tissue of 61 patients (n=45 for GC and n=16 for non-GC).
Results: There were included 137 relatives of GC patients and 34 of non-GC. The median age of the relatives of patients with a gastric tissue sample was 39 years (10-86). Infection was found in 60.2% (n=103) relatives. There were no higher H. pylori infection rates in relatives of patients with gastric cancer (62% vs 62.9%, p=0.33), H. pylori infection (60% vs 60%, p=0.96), or metaplasia (58.8% vs 61.8%, p=0.71).
Conclusion: The prevalence of infection in relatives of GC patients is high in our population but not associated with H. pylori presence in the paired case.
Introduction
Gastric cancer (GC) is the third most common cancer in Peru and the second most common cause of cancer deaths in the world [1-2]. Prevalence of precancerous gastric lesions and risk of developing GC is higher in relatives of patients with cancer [3]. Helicobacter pylori (H. pylori) is present in 50% of the world population [4], and infected patients have a higher risk of developing chronic gastritis (CG), atrophy, hypochloridria, and cancer of the distal and middle (non-cardia) part of the stomach [3,5]. Some strains of H. pylori are carriers of virulence genes and appear to have a greater carcinogenic potential [6]. Exposure to the same strains of H. pylori in relatives, and the fact that relatives share genes associated with predisposition to develop cancer in the presence of H. pylori infection, would indicate that first-degree relatives of patients with GC who are living in the same home require a more intense follow-up [6,5]. However, there is no information on the prevalence of H. pylori infection in relatives of patients with GC in Peru. The objective of the present study was to evaluate the relationship between the presence of H. pylori infection in Peruvian patients with GC and their relatives.
Materials and Methods
Study population
We included 61 patients domiciled in the Metropolitan Lima who came for diagnosis or screening to the National Institute of Neoplastic Diseases (INEN) from 2015 to 2018. Gastric sections were stored at -80°C. A total of 171 family members living in the same home were invited to have a urease breath test for the detection of H. pylori and absence of antibiotic treatment in the 2 previous weeks was requested (Figure 1).
Figure 1. Diagram of Study Population.
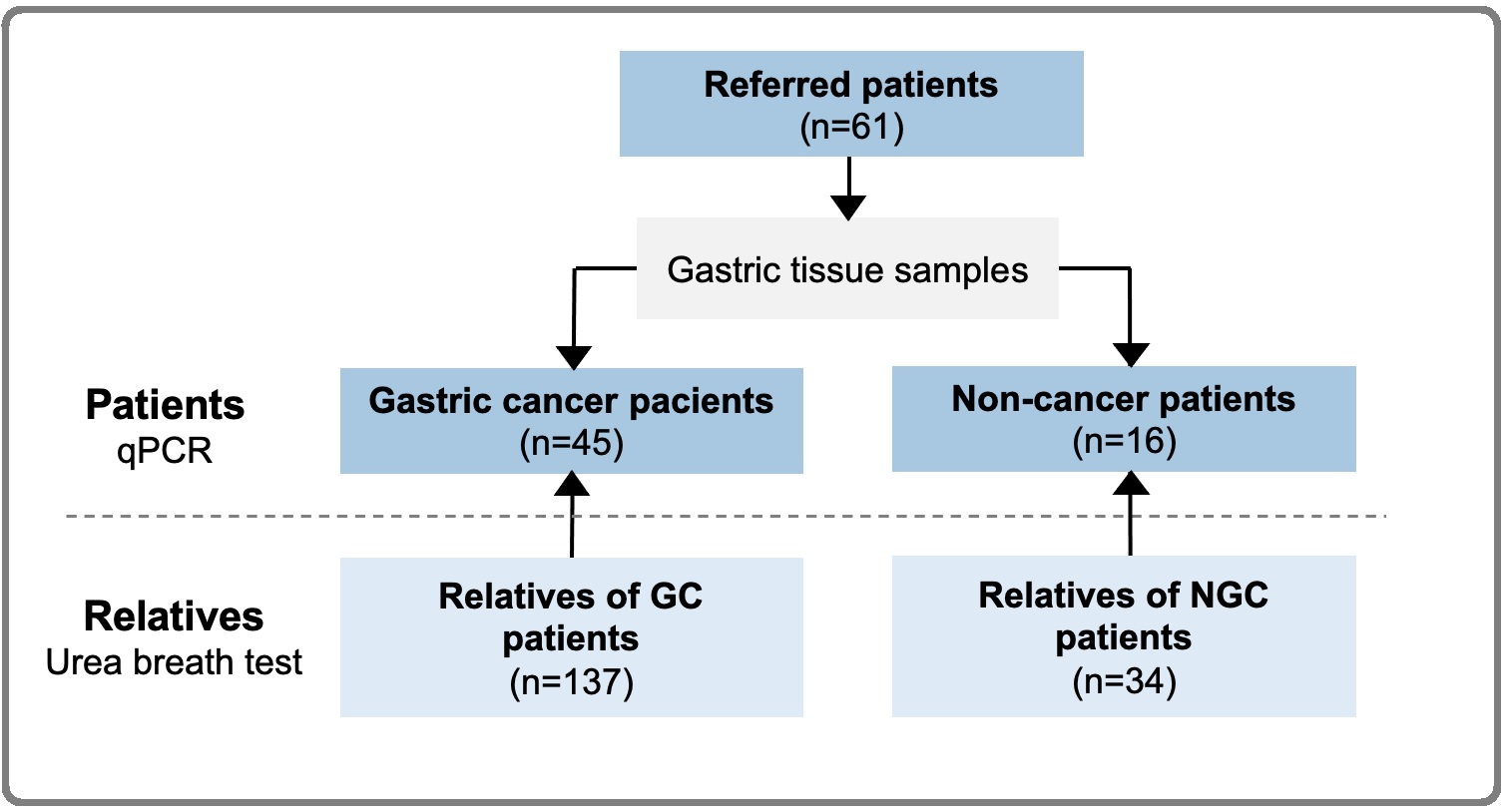
Patients and their relatives were surveyed about their socio-demographic factors. Relatives were classified as first, second, and third-degree kinship or couples. They were also grouped inside the family of the paired case with gastric tissue evaluation. Pathological gastric samples were prospectively evaluated for metaplasia following Sydney system [7] Finally, clinicopathological information was collected from the clinical records of patients with GC, and there was no follow-up for patients nor relatives. The study and informed consent were approved by Institutional Review Board and by the INEN Ethics Committee (# 050-2015-CIE / INEN).
Helicobacter pylori detection
The detection of H. pylori in gastric tissue samples was done through the extraction of genomic DNA with the GeneJETGenomic DNA kit (ThermoScientific, USA), quantification by Fluorometry using the fluorometerQubit (v2.0 InvitrogenbyLife Technologies, EEUU). Colonization (hspA and UreA) and virulence (cagA, vacAm and vacAs) genes in positive cases for hspA or UreA were detected by quantitative PCR (qPCR) in the LightCycler® 96 Instrument Thermal Cycler (ROCHE®). The primers used have been previously described [8]. The urea breath test for the diagnosis of H. pylori was performed using the non-invasive PY test method (C14). This quantitative test detects the presence of CO2 labeled with carbon-14 in the breath, after 20 minutes of ingesting a capsule containing urea labeled with carbon-14, which becomes the case of the presence of H. pylori in the intestinal light from where CO2 passes to the blood and then to the alveolar air. The results are presented in disintegrations per minute (DPM), being negative <40 DPM, indeterminate 41-49 DPM and positive> 50 DPM [9].
Statistic analysis
A descriptive analysis was performed through frequencies, percentages and summary measures. We used Pearson χ2 or Fisher’s exact tests to compare frequencies between groups, and. The statistical software IBM SPSS v.25 was used, and a value of p <0.05 was considered statistically significant.
Results
A total of 61 patients with gastric tissue samples (22 gastroscopy and 39 gastric resections) were included, 45 (73.8%) with GC (adenocarcinoma histology) and 16 (26.2%) without GC (chronic gastritis) (Table 1).
| Features | Patients | Relatives | ||
| n= 61 | % | n=171 | % | |
| Age | ||||
| Median (Range) | 59 (36-94) | 39 (10-86) | ||
| Sex | ||||
| Female | 29 | 47.5 | 97 | 56.7 |
| Male | 32 | 52.5 | 74 | 43.3 |
| Metaplasia (n=55) | ||||
| Yes | 29 | 52.7 | ||
| No | 26 | 47.3 | ||
| Gastric Cancer | ||||
| Yes | 45 | 73.8 | ||
| No | 16 | 26.2 | ||
| H. pylori infection | ||||
| Positive | 38 | 62.3 | 103 | 60.2 |
| Negative | 23 | 37.7 | 68 | 39.8 |
Most gastric cancer were undifferentiated (62%, 28/45) and most pathological stages were III (51.1%) and II (22.2%). Median age was 59 (36-94) years and most patients were male (52.5%). Concurrent Metaplasia was found in 52.7% (29/55), 24 GC patients and 5 non GC patients. H. pylori infection (determined by constitutive genes) was found in 62.3% (38/61). While 57.7% (26/45) of the patients with GC had the infection and 96.2% of them were positive for at least one of the cagA virulence genes (92.3%, 24/26), vacAm (61.5%, 16/26) and / or vacAs (80.8%, 21/26). We included 171 relatives, 137 relatives of patients with GC (a median of 4, 1-11 relatives) and 34 relatives of patients without GC (a median of 3, 1-9 relatives). Median age was 39 (10-86) years and 56.7% were female. The degree of kinship was 1-grade (58.5%), 2-grade (11.7%), 3-grade (12.9%) and couples (17%). The positivity for the breath test was 60,2% (103/171) in the total population and 62% (85/137) in the relatives of GC. Breath test was found positive in 61% (61/100) of 1-grade, 60% (12/20) of 2-grade, 54.5% (12/22) of 3-grade kinship and 62.1% (18/29) of couples (p=0.808). Positive in 62.5% (5/8) with GC sibling and 50% (1/2) with GC parent.
The prevalence of H. pylori infection in relatives (n=171) was not associated with H. pylori infection (60% vs 60%, p= 0.963), presence of intestinal metaplasia (58.8% vs 61.8%, p= 0.714) or with GC diagnosis (62% vs 52.9%, p= 0.332) in the gastric tissue of the paired patient (n=61) (Table 2).
| Features of patients | UBT result in Relatives | p | |
| Positive | Negative | ||
| n=103 (%) | n=68 (%) | ||
| Tissue with HP infection | |||
| Positive | 67 (59.3) | 44 (40.7) | 0.963 |
| Negative | 36 (60.0) | 24 (40.0) | |
| Malignant status of gastric tissue | |||
| Positive | 85 (62.0) | 52 (38.0) | 0.332 |
| Negative | 18 (52.9) | 16 (47.1) | |
| Concurrent Metaplasia | |||
| Yes | 53 (58.9) | 37 (41.1) | 0.714 |
| No | 42 (61.7) | 26 (38.3) |
UBT, urea breath test; HP, Helicobacter pylori
There was no association between H. pylori infection in at least one first-degree relative and H. pylori infection in the paired GC case (p=0.57).
Discussion
We found that the prevalence of infection in the relatives of the evaluated patients was 60.2%, however, a higher prevalence of H. pylori infection was not found in relatives of patients with GC, nor in relatives of patients with infected gastric tissue nor in relatives of patients with co-diagnosis of gastric metaplasia. This absence of association is probably due to the high prevalence of H. pylori infection in our population.
Different studies find that relatives of patients with GC have a higher risk of also developing the malignancy. Palli et al. evaluated 1016 patients with GC and 1623 controls without GC through questionnaires in Italy, and found that a maternal history of GC is the most significant contributory risk factor for GC in children. This association may be due to the fact that autosomal recessive genes with higher penetrance can be transmitted from mother to child, however it can also explain why mothers have prolonged contact with their children and share exposure to environmental agents such as H. pylori [10]. This last theory coincides with the finding that the risk of developing GC is higher in patients infected with H. pylori at an earlier age [3, 6, 11]. Czene et al. evaluated 9.6 million people included in the Swedish database that assesses the impact of genetic and environmental components on 15 common cancers. The study showed that environmental effect such as H. pylori infection, produce an increase in susceptibility to GC, due to the high correlation rate between spouses and a high correlation between siblings who shared childhood due to a close age range, while the genetic effect was very low [12].
Other studies find that prevalence of H. pylori infection is higher among relatives of GC patients and infection rates appears to be higher in family members with co-habitance during childhood like siblings. Thus, Chang et al. analyzed a total of 726 subjects, including 300 relatives of 300 patients with GC and 426 controls. They found that family members of patients with GC had a higher prevalence of H. pylori infection than controls (75.3% vs. 60.1%) evaluated by a high gastrointestinal endoscopic examination and urease test.
Individuals with a GC sibling (88.9%) or those with >1 GC family members (85.7%) had higher H. pylori infection rates than having a GC parent (64- 75%) [6]. Brenner et al. evaluated a total of 1351 people who participated in the German Health and Nutrition Survey, and found that the sero-prevalence of antibodies against H. pylori was much higher among children of patients with GC than in controls (69% vs 44%). Family history and H. pylori infection were associated with an increased risk of GC and the risk was higher in the presence of infection with cagA- positive H. pylori strain [11]. Finally, additive effect of infection and heritage was described by Shin and et al. who analyzed 428 patients with GC and 368 controls. They found that the risk of developing the malignancy was 3 times higher in those who had first degree relatives with GC. This effect was greater in the young group with H. pylori infection (5-fold risk increase) [13].
Different studies have evaluated the process in which H. pylori increases the GC risk in relatives of patients with GC. El-Omar et al. evaluated a total of 100 first-degree relatives of 40 patients with non-cardia GC, and found that relatives of patients with GC and coinfection with H. pylori (64%) have a higher prevalence of precancerous anomalies such as hypochlorhydria and atrophic gastritis [3]. Jablonska and Chlumska evaluated 108 first-degree relatives of patients with non-cardia GC patients and 73 controls with mild dyspepsia without relatives with cancer. They found that although the prevalence of infection was similar in relatives of patients with or without cancer, there was a much higher prevalence of intestinal atrophy and metaplasia in relatives of patients with GC. The eradication of H. pylori was associated with a reduction in the prevalence of atrophic gastritis [14]. Azuma et al. examined H. pylori infection and the HLA-DQA1 genotype in 82 patients with gastric adenocarcinoma and 167 unrelated controls. They found that the combination of absence of allele DQA1*O102 and H. pylori infection was associated with atrophic gastritis and intestinal type gastric adenocarcinoma. The analysis of serum pepsinogen indicated that its levels would mediate the development of atrophic gastritis [15]. A deficiency of our study is that the detection of H. pylori infection in relatives was carried out through the breath or urease test and not by anatomopathological study, as was performed in the patients. However, different studies indicate that the sensitivity and specificity of this non-invasive test is comparable to that of the pathological study [16].
These findings would suggest that the risk factors observed in low prevalence populations such as those described in European and Asian populations are different from that observed in populations of high prevalence of H. pylori infection such as Peru. In conclusion, H. Pylori infection rates in GC patients and their relatives are high. However, they were not related.
Acknowledgements
Conflicts of interest
The authors declare no conflicts of interest.
Funding declaration
This work was supported by InnovatePeru (#430-PNICP-PIAP-2014) and CONCYTEC (#197-2015-FONDECYT).
Ethical statement
The study was approved by the ethics committee of the Institutional of Instituto Nacional de Enfermedades Neoplasicas, approval no. 050-2015-CIE/INEN. Informed consent or substitute for it was obtained from all patients prior to the procedure.
References
- Cáncer gástrico en Lima metropolitana Pilco C, Payet M, Cáceres G. Rev Gastroenterol Peru.2006;26(4):377-385.
- Epidemiology of gastric cancer Rugge M, Fassan M, Graham DY. In 'Gastric Cancer', Eds Springer.2015;:23-24.
- Increased prevalence of precancerous changes in relatives of gastric cancer patients: Critical role of H. pylori El–Omar Emad M., Oien Karin, Murray Lilian S., El–Nujumi Adil, Wirz Angela, Gillen Derek, Williams Craig, Fullarton Grant, McColl Kenneth E.L.. Gastroenterology.2000;118(1). CrossRef
- Global burden of gastric cancer attributable toHelicobacterpylori Plummer Martyn, Franceschi Silvia, Vignat Jérôme, Forman David, de Martel Catherine. International Journal of Cancer.2014;136(2). CrossRef
- Helicobacter pylori infection and gastric histology in first-degree relatives of gastric cancer patients: a meta-analysis Rokkas Theodore, Sechopoulos Panos, Pistiolas Dimitrios, Margantinis Georgios, Koukoulis Georgios. European Journal of Gastroenterology & Hepatology.2010;22(9). CrossRef
- Role ofHelicobacter pylori infection among offspring or siblings of gastric cancer patients Chang Young-Woon, Han Yo-Seob, Lee Dong-Keun, Kim Hyo-Jong, Lim Hyun-Seok, Moon Jeong-Seop, Dong Seok-Ho, Kim Byung-Ho, Lee Joung-Il, Chang Rin. International Journal of Cancer.2002;101(5). CrossRef
- Classification and Grading of Gastritis Dixon Michael F., Genta Robert M., Yardley John H., Correa Pelayo. The American Journal of Surgical Pathology.1996;20(10). CrossRef
- Helicobacter pylori Genotyping from American Indigenous Groups Shows Novel Amerindian vacA and cagA Alleles and Asian, African and European Admixture Camorlinga-Ponce Margarita, Perez-Perez Guillermo, Gonzalez-Valencia Gerardo, Mendoza Irma, Peñaloza-Espinosa Rosenda, Ramos Irma, Kersulyte Dangeruta, Reyes-Leon Adriana, Romo Carolina, Granados Julio, Muñoz Leopoldo, Berg Douglas E., Torres Javier. PLoS ONE.2011;6(11). CrossRef
- Recurrencia de la infección gástrica con Helicobacter pylori en adultos peruanos con distrés postprandial dos años después de la erradicación exitosa Novoa Reyes I, Caravedo Martínez M, Huerta-Mercado Tenorio J, et al. . Rev Gastroenterol Peru.2014;34(1):15-21.
- Family history and risk of stomach cancer in Italy Palli D, Galli M, Caporaso NE, et al . Cancer Epidemiol Biomarkers Prev.1994;3(1):15-18.
- Helicobacter pylori infection among offspring of patients with stomach cancer Brenner Hermann, Bode Günter, Boeing Heiner. Gastroenterology.2000;118(1). CrossRef
- Environmental and heritable causes of cancer among 9.6 million individuals in the Swedish family-cancer database Czene Kamila, Lichtenstein Paul, Hemminki Kari. International Journal of Cancer.2002;99(2). CrossRef
- Stomach Cancer Risk in Gastric Cancer Relatives Shin Cheol Min, Kim Nayoung, Yang Hyo Jun, Cho Sung-Il, Lee Hye Seung, Kim Joo Sung, Jung Hyun Chae, Song In Sung. Journal of Clinical Gastroenterology.2010;44(2). CrossRef
- Genetic factors in the development of gastric precancerous lesions—a role of Helicobacter pylori ? Jablonská Markéta, Chlumská Alena. Journal of Physiology-Paris.2001;95(1-6). CrossRef
- The role of the HLA-DQA1 gene in resistance to atrophic gastritis and gastric adenocarcinoma induced byHelicobacter pylori infection Azuma Takeshi, Ito Shigeji, Sato Fukiko, Yamazaki Yukinao, Miyaji Hideki, Ito Yoshiyuki, Suto Hiroyuki, Kuriyama Masaru, Kato Takuji, Kohli Yoshihiro. Cancer.1998;82(6). CrossRef
- Invasive and non-invasive tests for Helicobacter pylori infection Vaira D., Holton J., Menegatti M., Ricci C., Gatta L., Geminiani A., Miglioli M.. Alimentary Pharmacology & Therapeutics.2000;14. CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2021
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- Xml downloaded - 0 times