Favourable Response to Chemotherapy in Ora-Located Intraocular Retinoblastoma Grade B, C, and D: A Case Series
Download
Abstract
Background: We aim to analyze which tumor location gives more favorable chemotherapy response in intraocular retinoblastoma grade B, C, and D as well as to report the first case series in Indonesia.
Methods: Six boys with age ranging from 10 weeks to 47 months old were recruited into the study from April 2019 to January 2020 at National Eye Centre, Cicendo Eye Hospital, and Hasan Sadikin General Hospital, Bandung, West Java, Indonesia. Retinoblastoma (RB) patients underwent examination under anesthesia (EUA). Tumor size and location were evaluated by using RetCam pre and post 2 cycles of chemotherapy. The tumors were classified according to the International Intraocular Retinoblastoma Classification.
Results: There were two patients with unilateral RB and four patients with bilateral RB. All patients had leukocoria and sought medical advice within 12 months of onset. There were one tumor in the macular zone, six tumors in the equatorial zone, and two tumors in the ora zone. Two cycles of intravenous vincristine, etoposide, and carboplatin (VEC) were administered and the tumor diameter was re-evaluated afterwards. The tumor size was decreased following 2 cycles of chemotherapy.
Conclusions: Ora zone showed a more favorable chemotherapy response.
Introduction
Retinoblastoma (RB) is a primary intraocular tumor in childhood with incidence of 1:15,000-20,000 live births and 5000-9000 new cases are diagnosed annually [1-2]. More than 90% of patients with RB live in developing countries with advanced clinical findings in the first encounter. Therefore, the mortality is high [3]. The prognosis of survival depends on early diagnosis and prompt treatment [2].
Classification of intraocular retinoblastoma (RB) is based on international retinoblastoma classification (IIRC) grade A to grade E [4]. The size and location of the tumor should be well-documented. The tumor size is based on the biggest base diameter measured with an indirect ophthalmoscope, ultrasound, or retina camera. The tumor location is classified into 3 zones: macula, equator, and ora. The macula is defined as a part of the retina between the superior and inferior temporal arteriole. The equator is located between the macula and equator zones, as estimated by the presence of vortex veins. The ora location includes the remaining anterior extent portion of the retina up to the ora serrata [5].
Chemoreduction is one of the modalities employed for intraocular RB. The goal is for tumor shrinkage before the administration of local therapy. About 10% of chemoreduction alone is effective but 90% of cases need combination with local therapy, both for intraocular and extraocular RB. The effect of chemoreduction will be significantly noted after 2 cycles; as it will reduce the tumor diameter by 35% and reduce the tumor thickness by 50% [6]. Shields et al. reported that from 54 tumors, the average tumor basal dimension was 12 μm and the average tumor thickness was 7 μm. After conducting chemoreduction for 2 months, 48 tumors (89%) showed calcification with the average basal dimension of 8 μm and the average tumor thickness of 4 μm. There was a reduction of basal tumor dimension by 35% and reduction of tumor thickness by 49% at the end of treatment [7]. A study conducted by Gombos et al. in intraocular RB patients who only received chemotherapy reported that systemic chemotherapy showed a better response in tumors located in the macula and tumors more than 2 μm in size. Chemotherapy response based on the tumor location is varied according to tumor grading. Tumor location will give a different prognosis in each patient [5].
Standard chemotherapy includes vincristine, etoposide, and carboplatin (VEC) protocol given at 28 days interval for six cycles [4]. Vincristine dose is 1.5 mg/m2 on day one (0.05 mg/kg for patients aged ≤36 months), etoposide 150 mg/m2 on day one and two (5 mg/kg for patients aged ≤36 months), carboplatin 560 mg/m2 on day one (18.6 mg/ kg for patients aged ≤36 months) [8].
We report the outcomes of six patients with 7 eyes and 10 tumors which underwent 2 cycles of chemotherapy, documented during examination under anaesthesia (EUA) by using RetCam. Five patients showed clinical improvement marked with tumor regression.
Materials and Methods
These case series were conducted from May 2019 to January 2020 in National Eye Centre, Cicendo Eye Hospital Bandung, and Dr. Hasan Sadikin Bandung General Hospital. All patients were male with age ranging from 10 weeks to 47 months old with presenting signs of leukocoria and presented to the hospital within 12 months after first recognition of the finding. No significant past medical history nor cancer history were identified in the family. History taking, physical examination, and ancillary tests were performed. Patients were diagnosed with intraocular RB grade B, C, and D based on the International Intraocular Retinoblastoma Classification. Written informed consent was obtained from the patients for publication of these case series and accompanying images. A copy of the written consent is available for review by the reviewer of this journal.
Results
Two patients had unilateral RB and four patients had bilateral RB. From ultrasound examination, all patients showed solid echogenic lesions suggesting intraocular masses. From EUA prior chemotherapy, 1 tumor in macula zone, 6 tumors in the equator zone, and 2 tumors in ora zone with size 173-823 μm, whereas the size ranged from 147 – 815 μm following chemotherapy. Patients received six cycles of VEC chemotherapy but for this case series we observed up to 2 cycles. Five patients showed good tumor regression while 1 patient passed away due to intracranial metastasis and from post-EUA chemotherapy showed vitreous seeding.
Discussion
This case series demonstrated an uncommon clinical finding of intraocular RB patients who showed a more favourable response to chemotherapy in the ora zone. All patients in this case series were boys which might be coincidental because RB has no gender predisposition. Retinoblastoma arises due to RB1 gene mutation through two-hit hypothesis [1,4]. We did not perform any genetic analysis on the patients. Leukocoria is a common clinical finding in retinoblastoma. Unfortunately, in developing countries, most of the patients presented in the hospital at an advanced stage [1, 4]. All patients in this case series presented with leukocoria. Patients underwent ancillary tests i.e., ultrasound examination showing solid echogenic lesions suggesting intraocular masses (Figure 1).
Figure 1. Ultrasound Imaging Suggesting of Intraocular Mass.
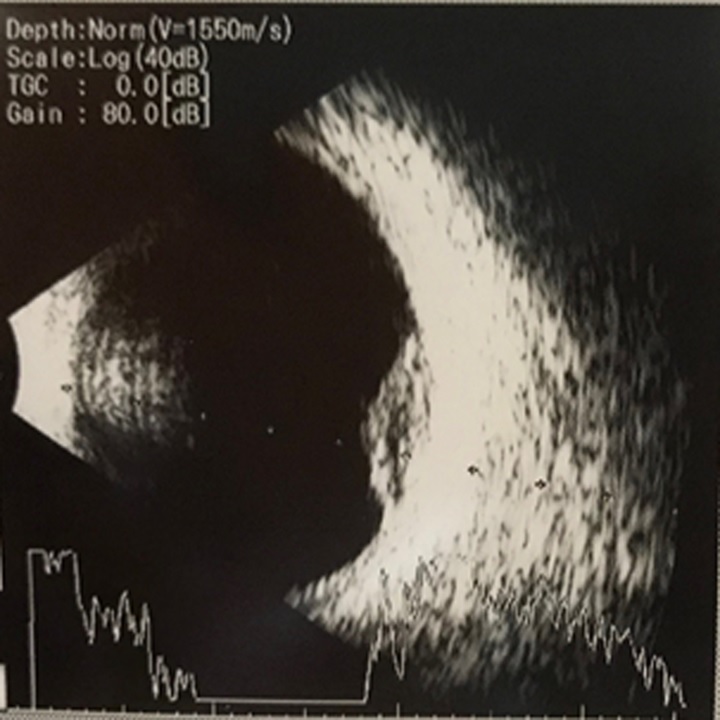
A previous study in the United Kingdom reported that most tumors are located in the macula zone compared to the equator or ora zone [5]. In this case series, we observed 1 tumor in the macula zone, 6 tumors in the equator zone, and 2 tumors in the ora zone. This is due to the previous epidemiology data obtained from developed countries; yet, we have not found epidemiology data from developing countries. The demographic differences could cause this finding. In developing countries, most of the patients presented at an advanced stage which is grade E. In this case series, 3 tumors were identified in one eye (equator zone). Another eye had two tumors in the ora zone but one of the tumor’s diameters could not be identified because the tumor size was beyond the visual field of EUA.
Based on the previous report, the greatest reduction of tumor diameter was reported in the macula zone. This is related to the fact that macula is well-vascularized so chemotherapy could reach the tumor optimally [5]. In this case series, reduction of tumor size ranging from 7 μm (tumor 8, equator zone) to 397 μm (tumor 6, ora zone). Due to the limited number of tumors being examined, the size reduction of tumors in macula could not be further analysed, unlike the previous study which showed a significant reduction of tumor size in the macula zone. The greatest reduction of tumor diameter was located in ora zone (tumor 6). From EUA performed after 2 cycles of chemotherapy, vitreous seeding is noted. The patient passed away on 27 December 2019 due to intracranial metastasis. The other five patients showed clinical improvement and tumor regression (Table 1).
| No | Tumor Location | Tumor diameter in millimeter (mm) | Difference (mm) | Percentage of tumor diameter reduction (%) | |
| Before chemotherapy | After 2 cycles of chemotherapy | ||||
| 1 | Equator | 437 | 395 | -42 | 9.6 |
| 2 | Macula | 173 | 159 | -14 | 8.1 |
| 3 | Equator | 823 | 815 | -8 | 0.9 |
| 4 | Ora | 384 | 364 | -20 | 5.2 |
| 5 | Equator | 328 | 237 | -91 | 27.7 |
| 6 | Ora | 640 | 243 | -397 | 62 |
| 7 | Equator (T1: Inferonasal) | 263 | 226 | -37 | 14.1 |
| 8 | Equator (T2: Superonasal) | 234 | 227 | -7 | 2.9 |
| 9 | Equator (T3: Superotemporal) | 231 | 147 | -84 | 36.4 |
mm, millimeters
A previous study suggested that tumors located in macula showed the best response to chemotherapy and after administration of 2 cycles chemotherapy tumor size will reduce by 35% [9]. However, in this case series, tumors located in ora showed a more favorable response to chemotherapy, and the percentage of reduction after 2 cycles chemotherapy ranging from 0.9% to 62% with an average of 18.54%. This finding could be affected by several factors, such as variation of response among Indonesian children, change of basal dimension, and the immeasurable tumor thickness.
In conclusion, the determination of tumor location and measurement of tumor size in patients with intraocular RB grade B, C, and D could help predicting the response to chemotherapy. Chemotherapy response is documented during evaluation. Ora zone has a more favorable response to chemotherapy.
Acknowledgments
Not applicable
List of abbreviations
Declarations
Availability of data and materials: All data are available without restriction. Researchers can obtain data by contacting the corresponding author.
Consent for publication
All patients were asked for the informed consent
Ethical approval and consent to participate
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the Ethics Committee of Dr. Hasan Sadikin General Hospital (protocol code LB.02.01/X.6.5/174/2019 on 29 May 2019).
Authors’ contributions
Conceptualization, A.S.; methodology, A.S., S.S., P.I., and F.K.; formal analysis, A.S.; investigation, A.S.; resources, A.S.; data curation, S.S., P.I., and F.K.; writing—original draft preparation, A.S.; writing—review and editing, S.S., P.I., and F.K.; supervision, S.S., P.I., and F.K. All authors have read and agreed to the published version of the manuscript.
Competing interests
The authors declare no competing interest in relation to this study.
References
- Retinoblastoma H Dimaras, K Kimani, Ea Dimba, P Gronsdahl, A White, Hs Chan, Bl Gallie. Lancet (London, England).2012;379(9824). CrossRef
- Retinoblastoma in Asia M Jain, D Rojanaporn, B Chawla, G Sundar, L Gopal, V Khetan. Eye (London, England).2019;33(1). CrossRef
- Retinoblastoma Dimaras Helen, Corson Timothy W., Cobrinik David, White Abby, Zhao Junyang, Munier Francis L., Abramson David H., Shields Carol L., Chantada Guillermo L., Njuguna Festus, Gallie Brenda L.. Nature Reviews. Disease Primers.2015;1. CrossRef
- Recent Developments in Retinoblastoma Rao Raksha, Honavar Santosh G.. The Official Scientific Journal of Delhi Ophthalmological Society.2016;27(1). CrossRef
- Retinoblastoma treated with primary chemotherapy alone: the significance of tumour size, location, and age Ds Gombos, A Kelly, Pg Coen, Je Kingston, Jl Hungerford. The British journal of ophthalmology.2002;86(1). CrossRef
- Chemoreduction in the management of intraocular retinoblastoma using new international classification Karkhaneh R, Pourmostadam B, Ahmadabadi MN. Iran J Ophthalmol.2010;22:31-5.
- Retinoblastoma management: advances in enucleation, intravenous chemoreduction, and intra-arterial chemotherapy Cl Shields, Ja Shields. Current opinion in ophthalmology.2010;21(3). CrossRef
- Systemic chemotherapy: a pediatric oncology perspective. In ‘Retinoblastoma’, Eds Ramasubramanian A and Shields CL Leahey AM. Jaypee Brothers Medical Publisher, New Delho.2012;:81-3.
- Chemotherapy in Retinoblastoma: Current Approaches Ö Yanık, K Gündüz, K Yavuz, N Taçyıldız, E Ünal. Turkish journal of ophthalmology.2015;45(6). CrossRef
License

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.
Copyright
© Asian Pacific Journal of Cancer Care , 2022
Author Details
How to Cite
- Abstract viewed - 0 times
- PDF (FULL TEXT) downloaded - 0 times
- XML downloaded - 0 times